University of Hyderabad (UoH) 2009 M.Sc Chemistry entrance - Question Paper
PART A
1. Which of the subsequent compounds has pyramidal geometry?
(A) B(CH3)3 (B) (CH3)3C+ (C) (CH3)3N (D) BF3
2. Which of the subsequent compounds exhibits optical isomerism?
(A) 1-Chloropentane (B) 1,5-Dichloropentane
(C) 3-Chloropentane (D) 2-Chloropentane
3. Identify the atom-economy reaction from the subsequent.
(A) Grignard reaction (B) Wittig reaction
(C) Diels-Alder reaction (D) Friedel-Crafts reaction
4. Chiral molecule B has [a] 20D= +24 for 40% optical purity with (R)-configuration. What will be [a] of the molecule B with (S)-configuration having 100% optical purity? 20D
(A) -30 (B) +30 (C) +60 (D) -60
5. The accurate order of stability of carbocations is:
(A) CH3+ > (CH3)2CH+ > CH3CH2+ > (CH3)3C+
(B) (CH3)3C+ > (CH3)2CH+ > CH3CH2+ > CH3+
(C) (CH3)3C+ > (CH3)2CH+ > CH3+ > CH3CH2+
(D) CH3CH2+ > CH3+ > (CH3)3C+ > (CH3)2CH+
6. The IUPAC name of is: OH
(A) Vinylethyl alcohol (B) 3-Ethylbut-3-en-1-ol
(C) 2- Ethylbut-3-en-1-ol (D) 2-Ethenylbutanol
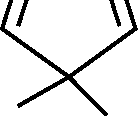
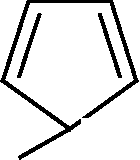
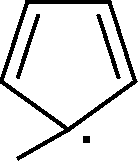
7. Which of the subsequent is aromatic?
(A)(B)(C)(D)HHHH.H..-+
8. The coordination number of a metal ion situated at the center of a square antiprism of ligand atoms is:
(A) two (B) four (C) six (D) 8
9. The strongest base among the subsequent is:
(A) AsH3 (B) PH3 (C) NH3 (D) SbH3
2
10. The qualitative test of "phosphate" is performed in the presence of an acid and
(A) Vanadate (B) Arsenate (C) Permanganate (D) Molybdate
11. Which of the subsequent is associated with the "layer type" structure?
(A) Graphite (B) Diamond (C) Fullerene (D) None of these
12. Which of the subsequent pairs cannot be used to produce hydrogen?
(A) Copper and hydrochloric acid (B) Sodium and ethanol
(C) Iron and sulfuric acid (D) Iron and steam
13. Which of the subsequent elements is associated with nitrogen fixing enzyme?
(A) Calcium (B) Nickel (C) Molybdenum (D) Cobalt
14. In eastern part of India and in Bangladesh many people are affected (skin symptom) by drinking water that contains the toxic element
(A) Hg (B) Sb (C) As (D) Se
15. For which of the subsequent sets of values of ?H and ?S will a reaction be spontaneous at 50o C ?
?H (kJ) ?S (J/K)
(A) +10 +30
(B) +10 - 30
(C) - 10 +30
(D) - 10 -30
16. Hg2+ + 2e- ? Hg E0 = 0.85 V
Zn2+ + 2e- ? Zn E0 = - 0.76 V
provided the cell potentials shown above, the overall cell potential for the subsequent reaction is
Zn + Hg2+ Zn2+ + Hg
(A) 0.09 V (B) 1.61 V (C) 0.80 V (D) 0.18 V
17. Half lifetime (t1/2) of a 2nd order reaction is proportional to (where A0 is the initial concentration of reactant)
(A) A0 (B) A02 (C) 1/A0 (D) Independent of A0
18. The equivalent conductance (ohm-1cm2eqv.-1) at infinite dilution (?0) of acetic acid (HAc) will be [Given ?0 (HCl) = 420, ?0 (NaCl) = 126 and ?0 (NaAc) = 91]:
(A) 385 (B) 637 (C) 455 (D) 203
3
19. 20 ml of 0.2 M NaOH and 40 ml of 0.1 M H2SO4 are mixed together in a standard flask and made up to 250 ml. The pH of the resulting solution is nearest to:
(A) five (B) six (C) seven (D) 8
20. Copper crystallizes in an fcc lattice with sides 3.61 Å. Atomic weight of copper is 63.54. The density of copper can be estimated as:
(A) 3.25 (B) 7.80 (C) 8.97 (D) 9.20
21. A shell leaves the gun barrel with a speed of 25 ms-1 at an angle of 45o from the horizon. Its trajectory (height vs horizontal distance travelled) is
(A) Straight line (B) Circle (C) Parabola (D) Hyperbola
22. The unit vector perpendicular to the plane described by the 2 vectors (i + j+ k) and
(i - j - k) is
(A) (i - j) /2 (B) (j - k) /2 (C) (k - i) /2 (D) (i - j + k) /3
23. If x2 + y2 + 4x - 6y + k = 0 represents a circle of radius five the value of k is
(A) 12 (B) -12 (C) 10 (D) -10
24. In the binary scale the number 55 is represented by
(A) 111001 (B) 110111 (C) 010101 (D) 101010
25. sin?p02 ? d? =
(A) 0 (B) p/4 (C) p/2 (D) p
4
PART B
26. The strongest acid among the subsequent is:
(A) CH3OH (B) CH3NH2
(C) CH3SH (D) CH3CH2NH2
27. The compound with least boiling point is
(A) 2-Methylhexane (B) 3,3-Dimethylpentane
(C) n-Heptane (D) Cycloheptane
28. Identify the fastest reacting compound in an SN2 reaction with OH ?.
(A) tert. Butyl chloride (B) Ethyl chloride
(C) 2,2-Dimethyl-1-propyl chloride (D) Isopropyl chloride
29. A compound that will provide 2 isomeric olefins on reaction with NaOMe will be:
(A) 1-Bromohexane (B) 3-Bromopentane
(C) Bromocyclohexane (D) 1-Phenyl-1-bromoethane
30. Identify the most improper reagent for the conversion of benzamide to benzylamine.
(A) NaBH4 (B) LiAlH4 (C) Pd/C/H2 (D) KH
31. Identify the most improper C-C bond forming reaction involving the carbocation intermediate.
(A) Cannizzaro reaction (B) Favorskii rearrangement
(D) Friedel-Crafts reaction (D) Benzoin condensation
32. Identify the achiral molecule from the subsequent.
(A) 2-Amino-2-carboxypropane (B) Alanine
(C) 2-Phenylpentane (D) Lactic acid
33. Identify the most improper product in the reaction of RCOOH with diazomethane.
(A) RCH2COOH (B) RCH2OH
(C) RCOOCH3 (D) RCOCH3
34. is an example of subsequent reaction type: CH
3CH2IN3-CH3CH2N3I-+
(A) SN1 (B) SN2 (C) SE1 (D) SE2
5
35. is an example of: Br Mg, ether
CO2
H+
1) COOH
2)
3)
(A) Kolbe reaction (B) Cannizzaro reaction
(C) Grignard reaction (D) Perkin condensation
36. Identify the alcohol which would be most easily dehydrated among the options provided.
(A) CH3CH2CH2CH2OH (B) CH3CH2CH(OH)CH3
(C) (CH3)2C(OH)CH2CH3 (D) (CH3)2CHCH(OH)CH3
37. The stability of formation of free radicals is in the subsequent order:
(A) Tertiary > Secondary > Primary > Methyl
(B) Tertiary > Primary > Secondary > Methyl
(C) Methyl > Tertiary > Secondary > Primary
(D) Methyl > Primary > Secondary > Tertiary
38. Identify the products that would be formed when acetophenone is reacted with I2 and NaOH.
(A) CH3COOH + PhI (B) PhCOONa + CH3I
(C) PhCOONa + CHI3 (D) PhCOONa + CH3COONa
39. The product of the subsequent reaction is:
OHCHCl3aq. NaOH+
(A) o-Hydroxybenzaldehyde (B) o-Chlorophenol
(C) Benzoquinone (D)p-Hydroxyphenol
40. The product formed when phthalic anhydride is treated with Zn/acetic acid is:
(A)(B)(C)(D)COOHCOOHCOOHOHOOO
41. What is the reagent used for the conversion of RCOCH2R' to RCOCOR'?
(A) H2O2 (B) SeO2 (C) OsO4 (D) HNO3/H2SO4
6
42. Sucrose on hydrolysis with diluted acids provide
(A) D(+)-Glucose and D(-)-Fructose (B) D(-)-Glucose and D(-)-Fructose
(C) D(+)-Glucose and L(-)-Fructose (D) two molecules of D(+)-Glucose
43. Predict the product of the subsequent reaction. OHOHOHCH3COOHZnCl2+
OHOHOHCOCH3(A)(B)OAcOAcAcO(C)OHOHOHCOCH3(D)OOOHOH
44. The degree of unsaturation of the fatty acid is determined by:
(A) Acid value (B) Iodine value
(C) Acetyl value (D) Reichert-Meissl value
45. The conversion of silver salt of the carboxylic acid to alkyl halide is called:
(A) Hunsdiecker reaction (B) Stephen reaction
(C) Ritter reaction (D) Vilsmeier reaction
46. Complete the subsequent nuclear reaction by identifying the missing product.
N + a ? ? + 11H 147
(A) 178O (B) ß- (C) ß+ (D) 168O
47. choose the group of ions corresponding to the larger ion from every pair.
[Co2+, Co3+], [Fe2+, Zn2+], [Na+, F-], [O2-, S2-]
(A) Co2+, Zn2+, F-, S2- (B) Co3+, Fe2+, Na+, S2-
(C) Co2+, Fe2+, F-, S2- (D) Co3+, Zn2+, Na+, O2-
48. Complete the sentence: An octahedral complex, MA4B2 ?????????? .
(A) Will have 2 constitutional isomers (B) Will have 2 stereoisomers
(C) Can not show isomerism (D) Will be optically active
49. Which 2 of the subsequent molecules/ions have planar structures?
(i) XeF4 (ii) ClO4- (iii) PtCl4- (iv) MnO4-
(A) i and iii (B) i and ii (C) ii and iii (D) ii and iv
7
50 When ammonium hydroxide is added to an aqueous solution of copper sulfate, the color of the solution becomes a deeper blue. The reaction taking place is best defined as:
(A) Redox (B) Rearrangement (C) Addition (D) Substitution
51. In acid medium 1 mole of Fe2+ will be equivalent to how many moles of MnO4-?
(A) five moles (B) 1/5 moles (C) two moles (D) 1/2 moles
52. The products found when chlorine reacts with cold and dilute solution of sodium hydroxide are:
(A) Cl- + ClO2- (B) Cl- + ClO-
(C) Cl- + ClO3- (D) Cl- + ClO4-
53. The oxidation states of boron in B2Cl4 and oxygen in hydrogen peroxide are, respectively,
(A) +2 and -2 (B) +3 and -1
(C) +2 and -1 (D) +3 and -2
54. 25.4 g of iodine and 14.2 g of chlorine are made to react completely to form a mixture of ICl and ICl3. The masses of ICl and ICl3 produced are:
(A) 16.25 g and 7.1 g respectively (B) 32.5 g and 7.1 g respectively
(C) 16.25 g and 23.35 g respectively (D) 25.4 g and 23.35 g respectively
55. organize the subsequent in the order of decreasing size: Ca2+, S2-, Ar, Cl-
(A) Cl-< S2-
(C) Ca2+< S2-< Ar< Cl- (D) Ca2+< Ar< Cl-
NaF, NaCl, LiCl, LiBr, LiI
(A) NaF, LiCl, NaCl, LiBr, LiI (B) LiI, LiBr, LiCl, NaCl, NaF
(C) NaF, NaCl, LiCl, LiBr, LiI (D) NaCl, NaF, LiI, LiBr, LiCl
57. organize the subsequent in the order of increasing boiling points: HF, HCl, HBr, HI
(A) HI < HCl < HBr < HF (B) HF < HBr < HCl < HI
(C) HCl < HBr < HI < HF (D) HCl < HF < HBr < HI
58. Among the following, which 2 do not contain sulfur?
Galena, Cassiterite, Stibnite, Rutile, Realgar, Cinnabar
(A) Stibnite and Rutile (B) Realgar and Cassiterite
(C) Galena and Cinnabar (D) Rutile and Cassiterite
8
59. Which of the subsequent is called the 3rd allotrope of carbon?
(A) Graphite (B) Fullerene (C) Diamond (D) Carbon nanotube
60. The Cubic unit cell is described by:
(A) a ? b ? c, a = ß = ? = 90o (B) a = b = c, a = ß = ? = 90o
(C) a = b ? c, a = ß = 90o, ? = 120o (D) a = b = c, a ? ß ? ?
61. In the compound [S2Mo5O23]4-, the oxidation state of sulfur is:
(A) 0 (B) +2 (C) +4 (D) +6
62. How many grams of magnesium sulfide are formed by reacting 0.20 moles of magnesium and 12.8 g of sulfur, if atomic weights of Mg and S are 24 and 32, respectively?
(A) 13.0 (B) 11.2 (C) 12.8 (D) 17.6
63. Which of the subsequent elements can exist in dry air without reacting?
(A) White P (B) Rb (C) Ca (D) Ag
64. The metal ion electronic configuration in high-spin octahedral [CoF6]3- is:
(A) t2g3eg3 (B) t2g4eg2 (C) t2g5eg1 (D) t2g6eg0
65. The metal ion that has zero magnetic moment is:
(A) Cu2+ (B) Cr3+ (C) V5+ (D) Mo5+
66. Solutions of the subsequent compounds, all at the identical molality, were prepared in a provided solvent. Which solution has the least freezing point?
(A) KBr (B) Al(NO3)3 (C) NaNO2 (D) MgCl2
67. A closed vessel maintained at a constant temperature of 0 oC contains a mixture of water and ice. The number of degrees of freedom available to the system is:
(A) 0 (B) one (C) two (D) 3
68. Units of the van der Waals' gas constants, a and b, are respectively:
(A) lit2 atm mole-2 and lit mole-1 (B) atm lit-2 mole2 and lit-1 mole
(C) atm-1 lit2 mole-1 and lit mole-1 (D) atm lit-1 mole2 and lit-1 mole
69. The Maxwell relation VTTPVS????????=????????implies that for a perfect gas
(A) (B) VRSln?VRTSln?
(C) 2VRTS? (D) PRTSln?
9
70. A 0.5 molal aqueous solution of glucose melts at 272.22 K. The melting point of a one molal solution of sucrose will be (Clue: melting point of ice is: 273.15 K):
(A) 271.29 K (B) 272.22 K (C) 269.43 K (D) 272.68 K
71. The charge of 0.4 mol of electron is equal to:
(A) – 5.79 × 104 C (B) – 1.00 × 104 C
(C) – 0.4 × 104 C (D) – 3.86 × 104 C
72. 6.5 mg of a hydrocarbon on combustion provide 11.2 ml of CO2 and 4.5 ml of water vapour at STP. The empirical formula of the hydrocarbon is:
(A) C5H8 (B) C5H6 (C) C5H4 (D) C5H2
73. The latent heat of melting of a solid is 330 J gm-1 at its melting point (300 K). The change in the entropy of a 2.0 gm sample when it melts at 300 K is:
(A) 0.55 JK-1 (B) 2.2 JK-1 (C) 0.45 JK-1 (D) 0.9 JK-1
74. The rate legal regulations for the single-step reaction, A + 2B ? C is:
(A) k [C] (B) k [C]/{[A] [B]2}
(C) k [A] [B]2 / [C] (D) k [A] [B]2
75. The heats of formation of CO and CO2 are – 110.5 and – 393.5 kJ/mol, respectively. The heat of reaction of CO + ½ O2 ? CO2 is
(A) -283 kJ/mole (B) -141.5 kJ/mole (C) + 141.5 kJ/mole (D) +283 kJ/mole
76. Boron doped silicon is:
(A) An intrinsic semiconductor (B) A p – kind semiconductor
(C) An n – kind semiconductor (D) A superconductor
77. The entropy change in an isolated system for a reversible process is:
(A) High (B) Low (C) Zero (D) Indeterminable
78. The product of the melting point and the entropy of fusion at constant pressure is called:
(A) Gibbs free energy (B) Enthalpy of fusion
(C) Helmholtz free energy (D) Specific heat
79. The bond dissociation energy is in the subsequent order:
(A) O – H > H – H > N – H > C – C
(B) C – C > N – H > H – H > O – H
(C) O – H > H – H > C – C > N – H
(D) C – C > O – H > H – H > N – H
10
80. Several metal oxides exist in nonstoichiometric state. In a sample having the formula TiO1.1, the ratio of Ti3+ / Ti2+ is:
(A) 0.10 (B) 0.25 (C) 0.33 (D) 0.67
81. If the first and second Balmer lines of the hydrogen atom appear at 1.523 × 104 and 2.056 × 104 cm-1. The third line should appear at:
(A) 2.216 × 104 cm-1 (B) 2.303 × 104 cm-1
(C) 2.504 × 104 cm-1 (D) 2.775 × 104 cm-1
82. The de Broglie wavelength of an electron (me = 9.109 × 10-31 Kg) traveling at a speed of 2.998 × 106 ms-1 is (given h = 6.626 × 10-34 Js):
(A) 1.215 × 10-10 m (B) 3.645 × 10-10 m
(C) 2.43 × 10-10 m (D) 4.86 × 10-10 m
83. The process of dispersion of a precipitate into colloidal state is called:
(A) Coagulation (B) Tyndall Effect
(C) Flocculation (D) Peptisation
84. A 0.001 M solution of a substance has an absorbance of 0.1 at a provided wavelength with a one cm pathlength cuvette. The molar extinction coefficient at this wavelength is:
(A) 10 M-1cm-1 (B) 100 M-1cm-1 (C) one M-1cm-1 (D) 1000 M-1cm-1
85. 2 compounds A and B, which interact reversibly to form a complex AB were mixed in a vessel at equal ratio. If the resulting concentration of every compound at the time of mixing is 0.21 M and after equilibrium is established the complex concentration is 0.2 M, the association constant Ka is:
(A) 2.0 × 100 M-1 (B) 2.0 × 101 M-1
(C) 2.0 × 102 M-1 (D) 2.0 × 103 M-1
86. dx/(16 - x?-222) =
(A) ln ¾ (B) three ln ¼ (C) ¼ ln three (D) four ln 1/3
87. The area of the triangle with vertices P(2, -3, 1), Q(1, -1, 2), R(-1, 2, 3) is:
(A) 2/3 (B) 23 (C) 32 (D) 3/2
88. The complex number (1 –3i) in polar form reads
(A) two cis 5p/3 (B) two cis 0 (C) two cis 3p/3 (D) two cis p/3
89. If 2 distinct numbers are chosen randomly from the 1st 50 natural numbers, the probability that both numbers are divisible by six is:
(A) 4/175 (B) 4/25 (C) 16/625 (D) 16/1175
11
90. The value of the sum 4S=40rC 2r-r is:
(A) 16/81 (B) 4/9 (C) 9/4 (D) 81/16
91. Lim xsinx =
x ? 0
(A) 0 (B) eight (C) one (D) Does not exist
92. The rank of the matrix 443850413- is:
(A) 0 (B) one (C) two (D) 3
93. The graph of f(x) = sin x/(2-cos x) (-p = x = p) is (A) (B) (C) (D)
94. cos?x/x dx =
(A) two cosx + c (B) two sinx + c
(C) two secx + c (D) two cosecx + c
95. The inverse of the matrix 00ii is:
(A) 00ii (B) 00ii- (C) 00ii- (D) 00ii--
96. The number of real roots of the formula x3 - 2x2 + 2x = 0 is:
(A) 0 (B) one (C) two (D) 3
97. The function with at lowest 1 local minimum among the subsequent is:
(A) e-x2 (B) e-x (C) ex (D) ex2
12
98. A discontinuous function among the subsequent is:
(A) Sin x (B) Cos x (C) Tan x (D) ex
99. Consider a sphere and a cube of maximum quantity that can be cut out of the sphere. The ratio of the quantity of the sphere to that of the cube is:
(A) 1/2 (B) p/2 (C) 1/3 (D) p/3
100. If A = 11---ii, AAT =
(A) one (B) i (C) -1 (D) 0
TIME: 2 HOURS MAXIMUM MARKS: 100
UNIVERSITY OF HYDERABAD ENTRANCE EXAMINATION - 200X M. Sc. Chemistry
HALL TICKET NUMBER
INSTRUCTIONS
1. Write your HALL TICKET NUMBER in the space provided above and also in the OMR ANSWER SHEET given to you.
2. Make sure that pages numbered from 1 - 13 are present (excluding pages assigned for rough work).
3. There are 100 questions in this paper. All questions carry equal marks.
4. There is negative marking. Each wrong answer carries mark.
5. Answers are to be marked on the OMR answer sheet following the instructions provided there upon.
6. Hand over both the question paper booklet and OMR answer sheet at the end of the examination.
7. In case of a tie, the marks obtained in the first 25 questions (PART A) will be used to determine the order of merit.
8. No additional sheets will be provided. Rough work can be done in the space provided at the end of the booklet.
9. Calculators (non-programmable) are allowed.
+
(A) B(CH3)3
(B) (CH3)3C
(C) (CH3)3N
Which of the following compounds exhibits optical isomerism?
(A) 1-Chloropentane (B) 1,5-Dichloropentane
(C) 3-Chloropentane (D) 2-Chloropentane
Identify the atom-economy reaction from the following.
(A) Grignard reaction (B) Wittig reaction
(C) Diels-Alder reaction (D) Friedel-Crafts reaction
Chiral molecule B has [a]0 = +24 for 40% optical purity with (R)-configuration. What will be [a]20 of the molecule B with (S)-configuration having 100% optical purity?
(A) -30
(C) +60
(B) +30
(D) -60
The correct order of stability of carbocations is:
(A) CH3+ > (CH3)2CH+ > CH3CH2+ > (CH3)3C+
(B) (CH3)3C+ > (CH3)2CH+ > CH3CH2+ > CH3+
(C) (CH3)3C+ > (CH3)2CH+ > CH3+ > CH3CH2+
(D) CH3CH2+ > CH3+ > (CH3)3C+ > (CH3)2CH+
,OH
(D)
 |
|
H |
H
The IUPAC name of
(A) Vinylethyl alcohol (C) 2- Ethylbut-3-en-1-ol
Which of the following is aromatic?
(A) (T\ (B)
H
 |
|
+ |
(B) 3-Ethylbut-3-en-1-ol (D) 2-Ethenylbutanol
(C)
is:
H
The coordination number of a metal ion situated at the center of a square antiprism of ligand atoms is:
(A) 2
(B) 4
(C) 6
9. The strongest base among the following is: (A) AsH (B) PH3 (C) NH3
10. The qualitative test of phosphate is performed in the presence of an acid and (A) Vanadate (B) Arsenate (C) Permanganate (D) Molybdate
11. Which of the following is associated with the layer type structure?
(A) Graphite (B) Diamond (C) Fullerene (D) None of these
12. Which of the following pairs cannot be used to produce hydrogen?
(A) Copper and hydrochloric acid (B) Sodium and ethanol
(C) Iron and sulfuric acid (D) Iron and steam
13. Which of the following elements is associated with nitrogen fixing enzyme?
(A) Calcium (B) Nickel (C) Molybdenum (D) Cobalt
14. In eastern part of India and in Bangladesh many people are affected (skin symptom) by drinking water that contains the toxic element
(A) Hg (B) Sb (C) As (D) Se
|
15. For which of the following sets of values of AH and AS will a reaction be spontaneous at 50o C ? | |||||||||||||||
|
16. Hg2+ + 2e- Hg E0 = 0.85 V Zn2+ + 2e- Zn E0 = - 0.76 V
Given the cell potentials shown above, the overall cell potential for the following reaction is
Zn + Hg2+ Zn2+ + Hg
(A) 0.09 V (B) 1.61 V (C) 0.80 V (D) 0.18 V
17. Half lifetime (t1/2) of a second order reaction is proportional to (where Ao is the initial concentration of reactant)
(A) A0 (B) A02 (C) 1/A0 (D) Independent of A0
18. The equivalent conductance (ohm-1cm2eqv.-1) at infinite dilution (A0) of acetic acid (HAc) will be [Given A0 (HCl) = 420, A0 (NaCl) = 126 and A0 (NaAc) = 91]:
(A)385 (B)637 (C)455 (D)203
19. 20 ml of 0.2 M NaOH and 40 ml of 0.1 M H2SO4 are mixed together in a standard flask and made up to 250 ml. The pH of the resultant solution is closest to:
(A) 5 (B) 6 (C) 7 (D) 8
20. Copper crystallizes in an fcc lattice with sides 3.61 A. Atomic weight of copper is 63.54. The density of copper can be estimated as:
(A) 3.25 (B) 7.80 (C) 8.97 (D) 9.20
21. A shell leaves the gun barrel with a speed of 25 ms-1 at an angle of 45o from the horizon. Its trajectory (height vs horizontal distance travelled) is
(A) Straight line (B) Circle (C) Parabola (D) Hyperbola
22. The unit vector perpendicular to the plane defined by the two vectors (i + j+ k) and (i - j - k) is
(A) (i - j)/V2 (B) (j - k) / V2 (C)(k - i) / V2 (D) (i - j + k) /V3
23. If x2 + y2 + 4x - 6y + k = 0 represents a circle of radius 5 the value of k is (A) 12 (B) -12 (C) 10 (D) -10
24. In the binary scale the number 55 is represented by
(A)111001 (B)110111 (C)010101 (D)101010
25. J sin2 9 d9 =
0
(A) 0 (B) n/4 (C) n/2 (D) n
26. The strongest acid among the following is:
(A) CH3OH (B) CH3NH2
(C) CH3SH (D) CH3CH2NH2
27. The compound with lowest boiling point is
(A) 2-Methylhexane (B) 3,3-Dimethylpentane
(C) n-Heptane (D) Cycloheptane
28. Identify the fastest reacting compound in an SN2 reaction with OH
(A) tert. Butyl chloride (B) Ethyl chloride
(C) 2,2-Dimethyl-1-propyl chloride (D) Isopropyl chloride
29. A compound that will give two isomeric olefins on reaction with NaOMe will be: (A) 1-Bromohexane (B) 3-Bromopentane
(C) Bromocyclohexane (D) 1-Phenyl-1-bromoethane
30. Identify the most appropriate reagent for the conversion of benzamide to benzylamine.
(A) NaBH4 (B) LiAlH (C) Pd/C/H (D) KH
31. Identify the most appropriate C-C bond forming reaction involving the carbocation intermediate.
(A) Cannizzaro reaction (B) Favorskii rearrangement
(D) Friedel-Crafts reaction (D) Benzoin condensation
32. Identify the achiral molecule from the following.
(A) 2-Amino-2-carboxypropane (B) Alanine (C) 2-Phenylpentane (D) Lactic acid
33. Identify the most appropriate product in the reaction of RCOOH with diazomethane.
(A) RCH2COOH (B) RCH2OH
(C) RCOOCH3 (D) RCOCH3
34. C H3CH2I - CH3CH2N3 + I is an example of following reaction type:
2) CO2
3) H
(A) Kolbe reaction (B) Cannizzaro reaction
(C) Grignard reaction (D) Perkin condensation
36. Identify the alcohol which would be most easily dehydrated among the choices given.
(A) CH3CH2CH2CH2OH (B) CH3CH2CH(OH)CH3 (C) (CH3)2C(OH)CH2CH3 (D) (CH3)2CHCH(OH)CH3
37. The stability of formation of free radicals is in the following order:
(A) Tertiary > Secondary > Primary > Methyl
(B) Tertiary > Primary > Secondary > Methyl
(C) Methyl > Tertiary > Secondary > Primary
(D) Methyl > Primary > Secondary > Tertiary
38. Identify the products that would be formed when acetophenone is reacted with I2 and NaOH.
(A) CH3COOH + PhI (B) PhCOONa + CH3I
(C) PhCOONa + CHI3 (D) PhCOONa + CH3COONa
39. The product of the following reaction is:
OH
aq. NaOH
+ CHCl,
'3
(A) o-Hydroxybenzaldehyde (B) o-Chlorophenol
(C) Benzoquinone (D)p-Hydroxyphenol
40. The product formed when phthalic anhydride is treated with Zn/acetic acid is:
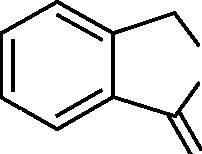
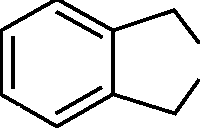
" OC - CC" -C"O ) O
O
41. What is the reagent used for the conversion of RCOCH2R to RCOCOR?
(A) H2O2 (B) SeO2 (C) OsO4 (D) HNO3/H2SO4
42. Sucrose on hydrolysis with diluted acids gives
(A) D(+)-Glucose and D(-)-Fructose (B) D(-)-Glucose and D(-)-Fructose
(C) D(+)-Glucose and L(-)-Fructose (D) 2 molecules of D(+)-Glucose
43. Predict the product of the following reaction.
ZnCL
+ CH3COOH
HO
OH
|
OH |
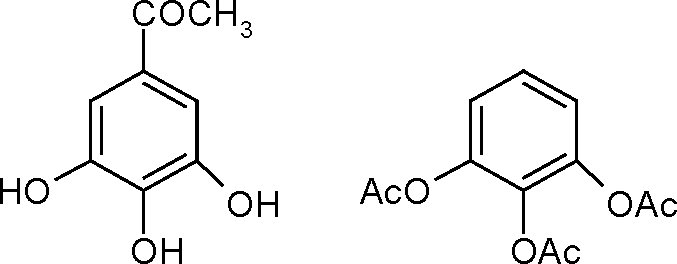 |
|
(A) (B) |
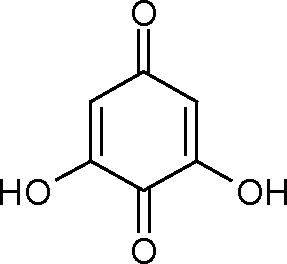 |
|
(D) |
(C)
COCH,
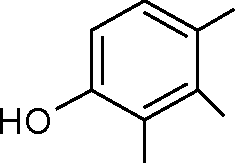 |
OH |
|
OH | |
44. The degree of unsaturation of the fatty acid is determined by:
(A) Acid value (B) Iodine value
(C) Acetyl value (D) Reichert-Meissl value
45. The conversion of silver salt of the carboxylic acid to alkyl halide is called:
(A) Hunsdiecker reaction (B) Stephen reaction
(C) Ritter reaction (D) Vilsmeier reaction
46. Complete the following nuclear reaction by identifying the missing product.
? + Jh
14
(B) P- (C) p+ (D) J8O
(A) o
47. Select the group of ions corresponding to the larger ion from each pair.
[Co2+, Co3+], [Fe2+, Zn2+], [Na+, F-], [O2, S2']
|
(A) Co2+, Zn2+, F-, S2- (C) Co2+, Fe2+, F-, S2- |
(B) Co3+, Fe2+, Na+, S2- (D) Co3+, Zn2+, Na+, O2- |
48. Complete the sentence: An octahedral complex, MA4B2__
(A) Will have two constitutional isomers (B) Will have two stereoisomers
(C) Can not show isomerism (D) Will be optically active
49. Which two of the following molecules/ions have planar structures?
(i) XeF4 (ii) ClO4- (iii) PtCU- (iv) MnO4-
(A) i and iii (B) i and ii (C) ii and iii (D) ii and iv
50 When ammonium hydroxide is added to an aqueous solution of copper sulfate, the color of the solution becomes a deeper blue. The reaction taking place is best described as:
(A) Redox (B) Rearrangement (C) Addition (D) Substitution
51. In acid medium one mole of Fe2+ will be equivalent to how many moles of MnO4-? (A) 5 moles (B) 1/5 moles (C) 2 moles (D) 1/2 moles
52. The products obtained when chlorine reacts with cold and dilute solution of sodium hydroxide are:
(A) Cl- + ClO2- (B) Cl- + ClO-
(C) Cl- + ClO3- (D) Cl- + ClO4-
53. The oxidation states of boron in B2Cl4 and oxygen in hydrogen peroxide are, respectively,
(A) +2 and -2 (B) +3 and -1
(C) +2 and -1 (D) +3 and -2
54. 25.4 g of iodine and 14.2 g of chlorine are made to react completely to form a mixture of ICl and ICl3. The masses of ICl and ICl3 produced are:
(A) 16.25 g and 7.1 g respectively (B) 32.5 g and 7.1 g respectively
(C) 16.25 g and 23.35 g respectively (D) 25.4 g and 23.35 g respectively
55. Arrange the following in the order of decreasing size: Ca2+, S2-, Ar, Cl-(A) Cl-< S2-<Ar< Ca2+ (B) Ca2+< Cl-< S2-< Ar
(C) Ca2+< S2-< Ar< Cl- (D) Ca2+< Ar< Cl-<S2-
56. Arrange the following in the order of increasing covalent character:
NaF, NaCl, LiCl, LiBr, LiI
(A) NaF, LiCl, NaCl, LiBr, LiI (B) LiI, LiBr, LiCl, NaCl, NaF
(C) NaF, NaCl, LiCl, LiBr, LiI (D) NaCl, NaF, LiI, LiBr, LiCl
57. Arrange the following in the order of increasing boiling points: HF, HCl, HBr, HI (A) HI < HCl < HBr < HF (B) HF < HBr < HCl < HI
(C) HCl < HBr < HI < HF (D) HCl < HF < HBr < HI
58. Among the following, which two do not contain sulfur?
Galena, Cassiterite, Stibnite, Rutile, Realgar, Cinnabar
(A) Stibnite and Rutile (B) Realgar and Cassiterite
(C) Galena and Cinnabar (D) Rutile and Cassiterite
59. Which of the following is called the third allotrope of carbon?
(A) Graphite (B) Fullerene (C) Diamond (D) Carbon nanotube
60. The Cubic unit cell is defined by:
(A) a b c, a = P = y = 90o (B) a = b = c, a = P = y = 90o
(C) a = b c, a = P = 90o, y = 120o (D) a = b = c, a P y
61. In the compound [S2Mo5O23]4-, the oxidation state of sulfur is:
(A) 0 (B) +2 (C) +4 (D) +6
62. How many grams of magnesium sulfide are formed by reacting 0.20 moles of magnesium and 12.8 g of sulfur, if atomic weights of Mg and S are 24 and 32, respectively?
(A) 13.0 (B) 11.2 (C) 12.8 (D) 17.6
63. Which of the following elements can exist in dry air without reacting?
(A) White P (B) Rb (C) Ca (D) Ag
64. The metal ion electronic configuration in high-spin octahedral [CoF6]3- is:
(A) t2g eg (B) t2g eg (C) t2g eg (D) t2g eg
65. The metal ion that has zero magnetic moment is:
(A) Cu2+ (B) Cr3+ (C) V5+ (D) Mo5+
66. Solutions of the following compounds, all at the same molality, were prepared in a given solvent. Which solution has the lowest freezing point?
(A) KBr (B) Al(NOs)3 (C) NaNO2 (D) MgCl2
67. A closed vessel maintained at a constant temperature of 0 oC contains a mixture of water and ice. The number of degrees of freedom available to the system is:
(A) 0 (B) 1 (C) 2 (D) 3
68. Units of the van der Waals gas constants, a and b, are respectively:
(A) lit2 atm mole-2 and lit mole-1 (B) atm lit-2 mole2 and lit-1 mole
(C) atm-1 lit2 mole-1 and lit mole-1 (D) atm lit-1 mole2 and lit-1 mole
69. The Maxwell relation I I = I I implies that for a perfect gas
\dV JT \dT )v
(A) S a Rln V (B) S a RTln V
70. A 0.5 molal aqueous solution of glucose melts at 272.22 K. The melting point of a 1 molal solution of sucrose will be (Clue: melting point of ice is: 273.15 K):
(A) 271.29 K (B) 272.22 K (C) 269.43 K (D) 272.68 K
71. The charge of 0.4 mol of electron is equal to:
(A) - 5.79 x 104 C (B) - 1.00 x 104 C
(C) - 0.4 x 104 C (D) - 3.86 x 104 C
72. 6.5 mg of a hydrocarbon on combustion gives 11.2 ml of CO2 and 4.5 ml of water vapour at STP. The empirical formula of the hydrocarbon is:
(A) C5H8 (B) C5H6 (C) C5H4 (D) C5H2
73. The latent heat of melting of a solid is 330 J gm-1 at its melting point (300 K). The change in the entropy of a 2.0 gm sample when it melts at 300 K is:
(A) 0.55 JK-1 (B) 2.2 JK-1 (C) 0.45 JK-1 (D) 0.9 JK-1
74. The rate law for the single-step reaction, A + 2B C is:
(A) k [C] (B) k [C]/{[A] [B]2}
(C) k [A] [B]2 / [C] (D) k [A] [B]2
75. The heats of formation of CO and CO2 are - 110.5 and - 393.5 kJ/mol, respectively. The heat of reaction of CO + V2 O2 CO2 is
(A) -283 kJ/mole (B) -141.5 kJ/mole (C) + 141.5 kJ/mole (D) +283 kJ/mole
76. Boron doped silicon is:
(A) An intrinsic semiconductor (B) A p - type semiconductor
(C) An n - type semiconductor (D) A superconductor
77. The entropy change in an isolated system for a reversible process is:
(A) High (B) Low (C) Zero (D) Indeterminable
78. The product of the melting point and the entropy of fusion at constant pressure is called:
(A) Gibbs free energy (B) Enthalpy of fusion
(C) Helmholtz free energy (D) Specific heat
79. The bond dissociation energy is in the following order:
(A) O - H > H - H > N - H > C - C
(B) C - C > N - H > H - H > O - H
(C) O - H > H - H > C - C > N - H
(D) C - C > O - H > H - H > N - H
80. Several metal oxides exist in nonstoichiometric state. In a sample having the formula TiO11, the ratio of Ti3+ / Ti2+ is:
(A) 0.10 (B) 0.25 (C) 0.33 (D) 0.67
81. If the 1st and 2nd Balmer lines of the hydrogen atom appear at 1.523 x 104 and 2.056 x 104 cm-1. The 3rd line should appear at:
(A) 2.216 x 104 cm-1 (B) 2.303 x 104 cm-1
(C) 2.504 x 104 cm-1 (D) 2.775 x 104 cm-1
82. The de Broglie wavelength of an electron (me = 9.109 x 10-31 Kg) traveling at a speed of 2.998 x 106 ms-1 is (given h = 6.626 x 10-34 Js):
(A) 1.215 x 10-10 m (B) 3.645 x 10-10 m
(C) 2.43 x 10-10 m (D) 4.86 x 10-10 m
83. The process of dispersion of a precipitate into colloidal state is called:
(A) Coagulation (B) Tyndall Effect
(C) Flocculation (D) Peptisation
84. A 0.001 M solution of a substance has an absorbance of 0.1 at a given wavelength with a 1 cm pathlength cuvette. The molar extinction coefficient at this wavelength is:
(A) 10 M-1cm-1 (B) 100 M-1cm-1 (C) 1 M-1cm-1 (D) 1000 M-1cm-1
85. Two compounds A and B, which interact reversibly to form a complex AB were mixed in a vessel at equal ratio. If the resultant concentration of each compound at the time of mixing is 0.21 M and after equilibrium is established the complex concentration is 0.2 M, the association constant Ka is:
(A) 2.0 x 10 M-1 (B) 2.0 x 101 M-1
(C) 2.0 x 102 M-1 (D) 2.0 x 103 M-1
86. j dx/(16 - x2) =
(A) ln % (B) 3 ln / (C) / ln 3 (D) 4 ln 1/3
87. The area of the triangle with vertices P(2, -3, 1), Q(1, -1, 2), R(-1, 2, 3) is:
(A) V2 /3 (B) 2 s (C) 3 V2 (D) V3 /2
88. The complex number (1 -V3i) in polar form reads
(A) 2 cis 5n/3 (B) 2 cis 0 (C) 2 cis 3n/3 (D) 2 cis n/3
89. If two distinct numbers are chosen randomly from the first 50 natural numbers, the probability that both numbers are divisible by 6 is:
(A) 4/175 (B) 4/25 (C) 16/625 (D) 16/1175
r=0
(A) 16/81 (B) 4/9
91. Lim xsinx =
x 0
(A) 0
(C) 1
(D) Does not exist
3 1 4 0 5 8 - 3 4 4
92. The rank of the matrix
is:
(A) 0 (B) 1 (C) 2 (D) 3
93. The graph of f(x) = sin x/(2-cos x) (-n < x < n) is
f(x) f(x) f(x)
f(x)
(A)
(B)
(C)
(D)
94. J cosVx/4x dx =
(A) 2 cos Vx + c (C) 2 secVx + c
95. The inverse of the matrix
(B) 2 sin Vx + c (D) 2 cosec Vx + c
0 i i 0
is:
|
0 i |
0 i |
0 - i |
0 - i | ||||
|
(A) |
i 0 |
(B) |
- i 0 |
(C) |
i 0 |
(D) |
- i 0 |
96. The number of real roots of the equation x3 - 2x2 + 2x = 0 is:
(A) 0 (B) 1 (C) 2 (D) 3
97. The function with at least one local minimum among the following is:
22 (A) e-x (B) e-x (C) ex (D) ex
98. A discontinuous function among the following is: (A) Sin x (B) Cos x (C) Tan x
99. Consider a sphere and a cube of maximum volume that can be cut out of the sphere. The ratio of the volume of the sphere to that of the cube is:
(D) ex
(C) 1/3
(D) n/3
(C) -1
(D) 0
|
(A) 1/2 |
(B) n/2 | ||
|
If A = |
1 |
- i |
, AAt |
|
- i |
-1 | ||
|
(A) 1 |
(B) i |
13
|
Attachment: |
| Earning: Approval pending. |
