Punjab University 2011 M.Sc Chemistry OCET - Question Paper
OCET 2011 Sr. No. :
ques. Booklet Series : A
Important : Please consult your Admit Card / Roll No. Slip before filling your Roll Number on the Test Booklet
and ans Sheet.
Roll No. In Figures In Words
O.M.R. ans Sheet Serial No.
Signature of the Candidate :
Subject : M. Sc. (Hons. School/2 Year Course)-Chemistry
Time : 90 minutes Number of ques. : 75 Maximum Marks : 75
DO NOT OPEN THE SEAL ON THE BOOKLET UNTIL ASKED TO DO SO
INSTRUCTIONS
1. Write your Roll No. on the ques. Booklet and also on the OMR ans Sheet in the space given
and nowhere else.
2. Enter the Subject and Series Code of ques. Booklet on the OMR ans Sheet. Darken the
corresponding bubbles with Black Ball Point / Black Gel pen.
3. Do not make any identification mark on the ans Sheet or ques. Booklet.
4. To open the ques. Booklet remove the paper seal (s) gently when asked to do so.
5. Please check that this ques. Booklet contains 75 ques.. In case of any discrepancy, inform the
Assistant Superintendent within 10 minutes of the begin of test.
6. every ques. has 4 option answers (A, B, C, D) of which only 1 is accurate. For every question,
darken only 1 bubble (A or B or C or D), whichever you think is the accurate answer, on the ans Sheet
with Black Ball Point / Black Gel pen.
7. If you do not want to ans a question, leave all the bubbles corresponding to that ques. blank in the
ans Sheet. No marks will be deducted in such cases.
8. Darken the bubbles in the OMR ans Sheet according to the Serial No. of the ques. provided in the
ques. Booklet.
9. Negative marking will be adopted for valuation i.e., 1/4th of the marks of the ques. will be deducted
for every wrong ans. A wrong ans means incorrect ans or wrong filling of bubble.
10. For calculations, use of simple log tables is permitted. Borrowing of log tables and any other material is not
allowed.
11. For rough work only the sheets marked "Rough Work" at the end of the ques. Booklet be used.
12. The ans Sheet is designed for computer valuation. Therefore, if you do not follow the instructions
provided on the ans Sheet, it may make valuation by the computer difficult. Any resulting loss to the
candidate on the above account, i.e., not subsequent the instructions completely, shall be of the
candidate only.
13. After the test, hand over the ques. Booklet and the ans Sheet to the Assistant Superintendent on duty.
14. In no case the ans Sheet, the ques. Booklet, or its part or any material copied/ noted from this
Booklet is to be taken out of the exam hall. Any candidate obtained doing so would be expelled from
the exam.
15. A candidate who creates disturbance of any type or modifications his/her seat or is obtained in possession of any
paper possibly of any assistance or obtained giving or receiving assistance or obtained using any other unfair
means during the exam will be expelled from the exam by the Centre Superintendent /
Observer whose decision shall be final.
16. Telecommunication equipment such as pager, cellular phone, wireless, scanner, etc., is not
permitted inside the exam hall. Use of calculators is not allowed.
1. The reactive intermediate carbene can be trapped by reaction with :
(A) Electrophile (B) Free radical
(C) Alkene (D) Nucleophile
2. The 1st step in photochemical chlorination of methane is :
(A) Homolytic cleavage of chlorine molecule
(B) Formation of carbanion
(C) Homolytic cleavage of methane
(D) Hetrolytic cleavage of chlorine
3. The decreasing order of stability in the subsequent set of carbanion is :
I. II. III.
(A) II > I > III (B) III > II > I
(C) II > III > I (D) I > II > III
4. Assign R / S configuration to the subsequent compound :
(A) 2S, 3R (B) 2R, 3R
(C) 2S, 3S (D) 2R, 3S
5. The number of configurational isomer in 2, 3-dibromo cinnamic acid is :
(A) three (B) 4
(C) two (D) 1
6. The conversion of 1-butene to 1-butanol can be achieved by :
(A) H2O, H2SO4 (B) B2H6, H2O2 / OH–
(C) Hg(OAc)2 / NaBH4 (D) HBr / KOH
7. Which of the subsequent alkene provide acetaldehyde and 3-pentanone upon ozonolysis ?
(A) 3-Ethyl-2-pentene (B) 3-Ethyl-1-pentene
(C) 2-Ethyl-2-pentene (D) 1-Ethyl-1-pentene
M. Sc. (Hons. School/2 Year Course)-Chemistry/A
CH2
O2N
–
CH2
– CH2
H3CO
–
COOH
OH
OH
COOH
H
H
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A three [Turn over
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 4
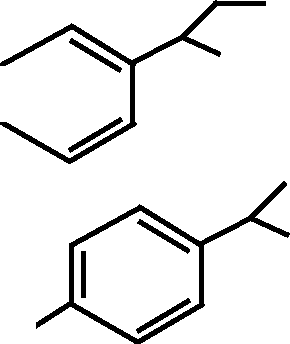
8. Free radical bromination of isopropyl benzene provide :
(A) (B)
(C) (D)
9. Aryl halides are less reactive as compared to alkyl halides toward nucleophilic substitution
due to :
(A) Inducitive effect (B) Mesomeric effect
(C) Field effect (D) Resonance stabilisation
10. Which of the compound react fastest with conc. HBr ?
(A) n-Propyl alcohol (B) iso-Propyl alcohol
(C) 2-Methyl-1-propanol (D) 2-Methyl-2-propanol
11. The reagent X and Y for the transformations provided beneath are :
(A) HI & NaOH (B) Sn / HCl & NaHCO3
(C) Zn / AcOH & H2 / Pd-C (D) Zn dust and Soda lime
12. The major product in the reaction provided beneath is :
(A) Benzaldehyde (B) Acetophenone
(C) Benzoic acid (D) Benzylalcohol
13. Reduction of carbonyl group to methylene i.e. (C = O ? CH2) can be achieved by :
(A) Wolf-Kishner reduction (B) MPV reduction
(C) NaBH4 reduction (D) Rousenmund reduction
Br Br
Br Br
S
S
Ph
H
I. n-BuLi/THF/–40oC
II. CH3l
III. Hg(ClO4)2 Product
COOH
COOH
OH
OH
X Y
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A five [Turn over
14. The accurate order of decreasing acidity subsequent carboxylic acid is :
I. II.
III. IV.
(A) I > III > II > IV (B) II > IV > I > III
(C) I > IV > II > III (D) III > IV > II > I
15. Acetic anhydride can be prepared by reaction of :
(A) Acetic acid with sodium acetate (B) Acetic acid with aluminum chloride
(C) Acetic acid with phosphorus pentaoxide (D) Acetic acid with ihionyl chloride
16. Separation of primary, secondary and tertiary amines can be achieved by :
(A) Hinsbergs reagent (B) Sangers reagent
(C) Bradys reagent (D) Tollens reagent
17. Reduction of benzonitrile (C6H5CN) with lithium aluminium hydride provide :
(A) Aniline (B) Benzyl amine
(C) o-Toludine (D) Benzamide
18. The pH at which amino acid behaves as neutral molecule is known as :
(A) Equivalent point (B) Isoelectric point
(C) Neutralization equivalent (D) Iodine number
19. Which of the subsequent carbohydrate is not a reducing sugar ?
(A) Glucose (B) Maltose
(C) Sucrose (D) Fructose
20. Which of the subsequent atom do not exhibit nuclear magnetic resonance ?
(A) N14 (B) C13
(C) P31 (D) F19
21. The region beneath 1500 cm–1 in infrared spectroscopy is known as :
(A) Far infrared region (B) Near infrared region
(C) Finger print region (D) Microwave region
22. The acidity of methylene protons in ethyl acetoacetate is due to :
(A) Inductive effect (B) Field effect
(C) Mesomeric effect (D) Resonance stabilisation
Br
COOH
F
COOH
Br COOH
Cl
COOH
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 6
23. Pyridine is less basic as compared to triethyl amine because lone pair resides in :
(A) sp hybride orbital (B) sp3 hybride orbital
(C) sp2 hybride orbital (D) p-orbital
24. The product X in the subsequent reaction is :
(A) 2-Methyl indole (B) 2-Phenyl indole
(C) 1-Phenyl-2-methyl indole (D) 1-methyl-2-phenyl indole
25. Reaction of methyl magnesium bromide (3 eq.) with diethyl carbonate followed by acidic
hydrolysis provide :
(A) 2-Methyl-2-propanol (B) 2-Propanol
(C) 1-Propanol (D) Propanal
26. The structure of beryllium chloride in the solid state is :
(A) Bridged dimer (B) Polymeric chain structure
(C) Linear (D) Tetrahedral
27. The element with atomic number 35 in the periodic table belongs to :
(A) s-block (B) p-block
(C) d-block (D) f –block
28. The geometry of XeOF2 :
(A) Pyramidal (B) Octahedral
(C) T-shaped (D) Tetrahedral
29. Alkyl lithium reacts with carbon dioxide to provide :
(A) Carboxylic acid (B) Alcohol
(C) Ketone (D) Esters
30. Which of the subsequent ion has highest enthalpy of hydration ?
(A) Li+ (B) Na+
(C) Rb+ (D) Cs+
31. The bond order in superoxide (O2)– ion is :
(A) two (B) 2.5
(C) 1.5 (D) 3
?????? ZnCl / 170o C
2
CH3 Ph
N
NH
X
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A seven [Turn over
32. The oxidation state of nitrogen in ammonium nitrate corresponds to :
(A) +3 (B) +5
(C) +3 and +5 (D) –3 and +5
33. In Wurtzite structure, Zn2+ ions occupy :
(A) All tetrahedral sites (B) Half tetrahedral sites
(C) All octahedral sites (D) Half octahedral sites
34. The state of hybridisation in interhalogen ion, ICl4
– is :
(A) sp3 hybridisation (B) sp3d hybridisation
(C) sp3d2 hybridisation (D) sp3d3 hybridisation
35. In the 1st transition series, the highest oxidation state is shown by :
(A) Cr (B) Co
(C) Cu (D) Mn
36. Ruthenium and osmium in the periodic table belong to :
(A) Cu (B) Mn
(C) Fe (D) Cr
37. Which of the subsequent has least oxidation state of the central atom ?
(A) Fe3[Fe(CN)6] (B) Na[Co(CO)4]
(C) Fe(CO)5 (D) [Co(en)3]Cl3
38. The coordination number of cerium in [Ce(NO3)4(Ph3PO)2]2– is :
(A) four (B) 6
(C) eight (D) 10
39. Which of the subsequent statements is not accurate for actinides and lanthanides ?
(A) Oxidation state of +3 is predominant in both the cases
(B) Both show contraction in their ionic radii
(C) The elements of both the series are radioactive
(D) Both involve the filling of f-orbitals
40. Cu+ disproportionates into :
(A) Cu only (B) Cu2+ and Cu3+
(C) Cu2+ and Cu (D) Cu and Cu–
41. Which of the subsequent is the strongest acid ?
(A) HClO4 (B) HClO3
(C) HClO2 (D) HOCl
42. AgCl is soluble in ammonium hydroxide due to the formation of :
(A) AgNH2 (B) AgCl.NH3
(C) [Ag(NH3)2Cl] (D) NH4[AgCl2]
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 8
43. The highest crystal field splitting will be for the ligand :
(A) C2O4
2– (B) NO2
–
(C) NH3 (D) CN–
44. Heme is a porphyrin complex of :
(A) Fe(II) (B) Fe(III)
(C) Mg(II) (D) Zn(II)
45. Ground state term of d five configuration is :
(A) 6S (B) 4F
(C) 2D (D) 3P
46. Which of the subsequent does not have bridging carbonyls ?
(A) Fe3(CO)12 (B) Fe2(CO)9
(C) Co4(CO)12 (D) Ru3(CO)12
47. Which of the subsequent is not an organometallic compound ?
(A) Pb(C2H5)4 (B) Fe(C5H5)2
(C) Si(OC2H5)4 (D) Sn(C4H9)4
48. Which of the provided complex does not provide precipitate of silver chloride on addition of
silver nitrate ?
(A) CoCl3.6NH3 (B) CoCl3.5NH3
(C) CoCl3.4NH3 (D) CoCl3.3NH3
49. Which of the subsequent is not a hard acid ?
(A) Na+ (B) Mg2+
(C) Ti4+ (D) Hg2+
50. The colour of copper sulphide is :
(A) Red (B) Yellow
(C) Black (D) Blue
51. What will be the energy (in eV) of an electron in ground state constrained to move in an
infinite 1 dimensional box of width 1Å ?
(A) 38 eV (B) 152 eV
(C) 19 eV (D) 342 eV
52. The Hooks legal regulations potential of an Simple Harmonic Oscillator is :
(A) A circle (B) An ellipse
(C) A parabola (D) A hyperbola
53. The formula for the Lamberts legal regulations is :
(A) ln (I0 / I) = –bx (B) ln (I / I0) = –bx
(C) ln (I / I0) = –?Cx (D) ln (I / I0) = ?Cx
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A nine [Turn over
54. Which of the subsequent molecule is IR-inactive but Raman-active ?
(A) Protein (B) HBr
(C) H2O (D) N2
55. A compound of Xe and F is obtained to have 53.5% of Xe. What is the oxidation state of Xe
in this compound ?
(A) –4 (B) 0
(C) +4 (D) +6
56. Amount of heat needed to change 1g ice at 0oC to 1g steam at 100o C is :
(A) 616 cal (B) 12 kcal
(C) 717 cal (D) 919 cal
57. In the limit T ? 0, for a crystal
(A) ST = CP/2 (B) ST = CP/3
(C) ST = CP/4 (D) ST = CP
58. compute the enthalpy of hydration of anhydrous copper sulphate (CuSO4) into hydrated
copper sulphate (CuSO4.5H2O). provided that the enthalpies of solutions of anhydrous copper
sulphate and hydrated copper sulphate are –66.5 and +11.7 kJ/mol respectively.
(A) –78.2 kJ/mol (B) –54.8 kJ/mol
(C) +54.8 kJ/mol (D) +78.2 kJ/mol
59. The electronic partition function of an atom whose atomic state is 2D3/2 is :
(A) 3/2 (B) 3
(C) four (D) 2/3
60. The distance travelled by an ion per 2nd under a potential gradient of one volt per cm is
called :
(A) Ionic gradient (B) Ionic mobility
(C) Ionic potential (D) Ionic conductance
61. The pH of a solution is enhanced from two to 3. The concentration of H+ in the new solution
(A) is 3 times the original solution (B) is about 1.5 times the original solution
(C) Increases 10 times (D) reduces 10 times
62. The standard reduction potentials in volts for Pb2+ and Ag+ are –0.13 and +0.80
respectively. compute Eo in volts for a cell in which the overall reaction is
Pb + two Ag+? Pb2+ + two Ag :
(A) 0.93 (B) 0.67
(C) 1.73 (D) 1.47
63. A crystal having unit cell dimensions a ? b ? c, a = ß = ? = 90o is :
(A) Cubic (B) Tetragonal
(C) Monoclinic (D) Orthorhombic
64. The edge length of face centered unit cubic cell is 508 pm. If the radius of the cation is
110 pm, radius of the anion is :
(A) 144 pm (B) 288 pm
(C) 618 pm (D) 398 pm
65. The value of van der Waals constant a for hydrogen gas when critical temperature is
33.2oC and its critical pressure is 12.4 atm :
(A) 24.912 atm litre2 mol–1 (B) 21.439 atm litre2 mol–1
(C) 47.935 atm litre2 mol–1 (D) 37.428 atm litre2 mol–1
66. A gas will approach ideal behaviour at :
(A) Low temp and low pressure (B) Low temp and high pressure
(C) High temp and low pressure (D) High temp and high pressure
67. Which of the subsequent pairs of solutions will be isotonic at the identical temperature ?
(A) 0.1 m glucose and 0.1 m KCl (B) 0.1 m glucose and 0.1 m MgCl2
(C) 0.1 m K2SO4 and 0.1 m KCl (D) 0.1 m Na2SO4 and 0.1 m Ca(NO3)
68. The units in which surface tension is measured :
(A) Dyne cm (B) Dyne cm–1
(C) Dyne–1 cm (D) Dyne–1 cm–1
69. The half life period for catalytic decomposition of AB3 at 50 mm is four hrs and at 100 mm it
is two hrs. The order of the reaction is :
(A) Zero (B) 1
(C) two (D) 3
70. The replaced distribution legal regulations for the solute undergoing dissociation in 1 of the solvents
is :
(A) KD = C1 / vC2 (B) KD = C1 / C2 (1 – a)
(C) KD = C1 / C2 (a – 1) (D) KD = C1 / C2
71. The decomposition of CaCO3 in a closed vessel is represented by the formula
CaCO3 (s) ? CaO (s) + CO2 (g)
The number of phases and components respectively are :
(A) three and two (B) two and 3
(C) two and two (D) three and 3
72. The activation energy of a reaction can be determined from the slope of which of the
subsequent graphs :
(A) ln k vs T (B) ln k / T vs 1/T
(C) T / ln k vs 1/T (D) ln k vs 1/T
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 10
73. The function of alum used for the purification of water is to :
(A) Coagulate the sol particles (B) Disperse the sol particles
(C) Emulsify the sol particles (D) Absorb the sol particles
74. Freundlich isotherms is not applicable at :
(A) Room temperature (B) Low pressure
(C) 273 K (D) High pressure
75. The osmotic pressure in millimetres of mercury at 15oC of a solution of naphthalene
(C10H8) in benzene containing 14g of naphthalene per litre of solution :
(A) 2.586 mm (B) 1965 mm
(C) 262 mm (D) 199037 mm
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 11
ROUGH WORK
M. Sc. (Hons. School/2 Year Course)-Chemistry/OEC-22971-A 12 211
Sr. No. :
Important : Please consult your Admit Card / Roll No. Slip before filling your Roll Number on the Test Booklet
and Answer Sheet.
In Words
Roll No.
In Figures
0.M.R. Answer Sheet Serial No. _|_
Signature of the Candidate : _
Subject : M. Sc. (Hons. School/2 Year Course)-Chemistry
Time : 90 minutes Number of Questions : 75 Maximum Marks : 75
DO NOT OPEN THE SEAL ON THE BOOKLET UNTIL ASKED TO DO SO INSTRUCTIONS
1. Write your Roll No. on the Question Booklet and also on the OMR Answer Sheet in the space provided and nowhere else.
2. Enter the Subject and Series Code of Question Booklet on the OMR Answer Sheet. Darken the corresponding bubbles with Black Ball Point / Black Gel pen.
3. Do not make any identification mark on the Answer Sheet or Question Booklet.
4. To open the Question Booklet remove the paper seal (s) gently when asked to do so.
5. Please check that this Question Booklet contains 75 questions. In case of any discrepancy, inform the Assistant Superintendent within 10 minutes of the start of test.
6. Each question has four alternative answers (A, B, C, D) of which only one is correct. For each question, darken only one bubble (A or B or C or D), whichever you think is the correct answer, on the Answer Sheet with Black Ball Point / Black Gel pen.
7. If you do not want to answer a question, leave all the bubbles corresponding to that question blank in the Answer Sheet. No marks will be deducted in such cases.
8. Darken the bubbles in the OMR Answer Sheet according to the Serial No. of the questions given in the Question Booklet.
9. Negative marking will be adopted for evaluation i.e., 1/4th of the marks of the question will be deducted for each wrong answer. A wrong answer means incorrect answer or wrong filling of bubble.
10. For calculations, use of simple log tables is permitted. Borrowing of log tables and any other material is not allowed.
11. For rough work only the sheets marked Rough Work at the end of the Question Booklet be used.
12. The Answer Sheet is designed for computer evaluation. Therefore, if you do not follow the instructions given on the Answer Sheet, it may make evaluation by the computer difficult. Any resultant loss to the candidate on the above account, i.e., not following the instructions completely, shall be of the candidate only.
13. After the test, hand over the Question Booklet and the Answer Sheet to the Assistant Superintendent on duty.
14. In no case the Answer Sheet, the Question Booklet, or its part or any material copied/ noted from this Booklet is to be taken out of the examination hall. Any candidate found doing so would be expelled from the examination.
15. A candidate who creates disturbance of any kind or changes his/her seat or is found in possession of any paper possibly of any assistance or found giving or receiving assistance or found using any other unfair means during the examination will be expelled from the examination by the Centre Superintendent / Observer whose decision shall be final.
16. Telecommunication equipment such as pager, cellular phone, wireless, scanner, etc., is not permitted inside the examination hall. Use of calculators is not allowed.
M. Sc. (Hons. School/2 Year Course)-Chemistry/A
The reactive intermediate carbene can be trapped by reaction with :
(A) Electrophile (B) Free radical
(C) Alkene (D) Nucleophile
The first step in photochemical chlorination of methane is :
2.
(A) Homolytic cleavage of chlorine molecule
(B) Formation of carbanion
(C) Homolytic cleavage of methane
(D) Hetrolytic cleavage of chlorine
The decreasing order of stability in the following set of carbanion is :
3.
CH
Q
CH
CH

a
H3CO
I.
II.
III.
III > II > I I > II > III
(B)
(D)
(A) II > I > III (C) II > III > I
O2N
4. Assign R / S configuration to the following compound :
COOH OH
H-
H
OH
COOH
(A) 2S, 3R (B) 2R, 3R
(C) 2S, 3S (D) 2R, 3S
The number of configurational isomer in 2, 3-dibromo cinnamic acid is :
5.
(A) 3 (B) 4
(C) 2 (D) 1
6. The conversion of 1-butene to 1-butanol can be achieved by :
(A) H2O> H2SO4 (B) B2H65 H2O2 / OH_
(C) Hg(OAc)2 / NaBH4 (D) HBr / KOH
7. Which of the following alkene gives acetaldehyde and 3-pentanone upon ozonolysis ?
(A) 3-Ethyl-2-pentene (B) 3-Ethyl-1-pentene
(C) 2-Ethyl-2-pentene (D) 1-Ethyl-1-pentene
Br
 |
|
Br |
(B)
(D)
8. F ree radical bromination of isopropyl benzene gives :
Br
H H
(A)
(C)

9. Aryl halides are less reactive as compared to alkyl halides toward nucleophilic substitution due to :
(A) Inducitive effect (B) Mesomeric effect
(C) Field effect (D) Resonance stabilisation
10. Which of the compound react fastest with conc. HBr ?
(A) n-Propyl alcohol (B) iso-Propyl alcohol
(C) 2-Methyl-1-propanol (D) 2-Methyl-2-propanol
11. The reagent X and Y for the transformations given below are :
|
COOH OH |
 |
|
OH |
|
(A) HI & NaOH (C) Zn / AcOH & H2 / Pd-C |
(B) Sn / HCl & NaHCO3 (D) Zn dust and Soda lime |
12. The major product in the reaction given below is :
I. n-BuLi/THF/-40oC
II. CH
III. Hg(ClO4)2 ,
c*
Ph
H
Product
(A) Benzaldehyde (B) Acetophenone
(C) Benzoic acid (D) Benzylalcohol
13. Reduction of carbonyl group to methylene i.e. (C = O CH2) can be achieved by :
(A) Wolf-Kishner reduction (B) MPV reduction
(C) NaBH4 reduction


