Acharya Nagarjuna University (ANU) 2007-2nd Year M.Sc Chemistry - - Question Paper
Chemistry - second year Full set
(DCHE 21)
M.Sc. (Final) DEGREE EXAMINATION,
DECEMBER 2007.
Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks
PART A (4 x 10 = 40 marks)
Answer any FOUR questions.
1. Describe the instrumentation of Turbidimetry and its working principle.
Mention few applications of this technique.
2. What is Qualitative and Quantitative analysis? Explain the method of
determining Iron by using spectrophotometer.
3. What are the important components of spectrofluorimeter? Explain its
working principle.
4. Write the applications of Atomic Absorption Spectrometer (AAS).
5. What are red-ox titrations? Explain the usefulness of potentiometric
titrations.
6. Describe the electrogravimetric technique with suitable examples.
7. Explain the solvent extraction principle and mention its advantages.
8. Describe the principle of thin layer chromatographic technique.
PART B (4 x 15 = 60 marks)
Answer ALL questions.
State and explain Beers Law. Describe method of determining manganese and phosphate by using spectrophotometer.
Explain the instrumentation and applications of Infrared Spectroscopy.
10. (a) What are the important components of Atomic Absorption
Spectrometer? Explain its working principle.
Or
(b) Describe the working of Flame photometer. Write few applications of this instrument.
11. (a) What are conductometric titrations? Draw and explain the strong acid and strong base neutralisation curve.
Or
(b) Explain the usefulness of potentiometer in the precipitation and complexometric reactions with suitable examples.
12. (a) What are the important components of Gas-Liquid chromatography? Explain its working principle.
Or
(b) What are Ion-exchangers? Explain the Ion-exchange mechanism for the separation of different types of ions.
M.Sc. (Final) DEGREE EXAMINATION
DECEMBER 2007.
Second Year
Chemistry
Paper VI INORGANIC CHEMISTRY Time : Three hours Maximum : 100 marks
PART A (4 x 10 = 40 marks)
Answer any FOUR questions.
1. Give an account on extraction and separation of lanthanides.
2. Write about the uses of lanthanides and actinides.
3. How do you elucidate the structures of metal complexes using electronic
absorption spectroscopy?
4. Describe the principle and instrumentation of Raman spectroscopy.
5. Discuss the principle and instrumentation of NMR spectroscopy.
6. Explain the significance of g factor.
7. Give a brief account on metal complexes in medicine and concept of
essentiality.
8. Write about the synthetic oxygen carriers.
PART B (4 x 15 = 60 marks)
Answer ALL questions.
(a) Discuss the magnetic properties and spectral properties of actinides.
9
10
11.
Or
(b) What are the consequences of lanthanide contraction? How
lanthanide contraction effects the chemical properties of lanthanides?
12. (a) Explain the role and mechanism of nitrogenase enzyme in biological nitrogen fixation.
Or
(b) Give the classification of essential elements and concept of essentiality. Explain the metal ion toxicity.
M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2007
Second Year Chemistry Paper VII ORGANIC CHEMISTRY
Time : Three hours
Maximum : 100 marks
PART A (4 x 10 = 40 marks)
Answer any FOUR questions.
1. (a) Define the terms Bathochromic shift and Hyperchromic shift.
(5)
(5)
(5)
(5)
(b) Define Beer Lambert's law and explain the terms involved.
(a) What are the vibrational modes in IR.
2.
(b) How do distinguish the following isomers by using IR spectroscopy.
|
(i) | 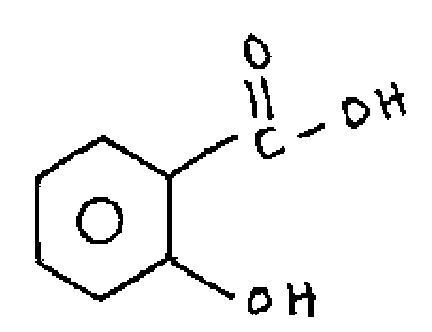 |
|
(ii) | 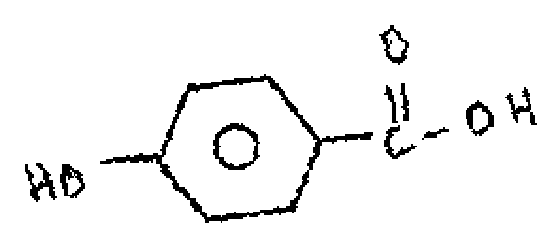 |
(2)
(i)
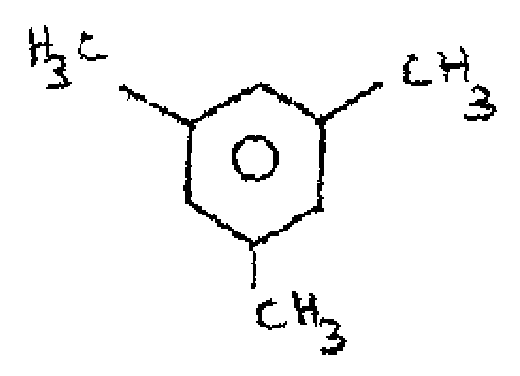 |
(ii) |
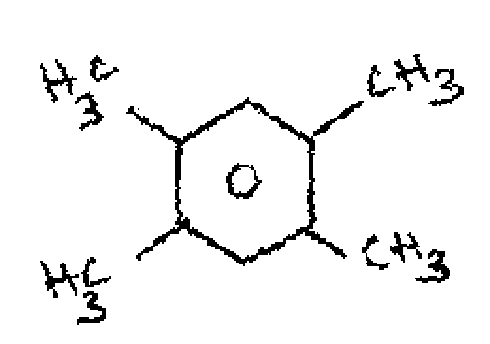
(c) Define chemical shift and Geminal coupling.
(5)
4. (a) Write the types of cleavages in mass spectrum.
(b) How do distinguish 1, 2 and 3 -alcohols by using mass spectrum. (5)
(b) Write a short notes on Barton reaction.
6. Write the wood Word-Haffmann diagrams for [4 + 2] cyclo addition reactions.
(5)
(10)
(5)
(5)
(5)
(5)
(10)
(5)
7.. (a) Define alkaloid and give any synthesis of Nicotine.
(b) Write the structural elucidation of a-terpeneol.
Discuss the mechanisms for the following reactions 8.. (a) Neber rearrangement.
(b) Demjanov rearrangement.
PART B (4 x 15 = 60 marks)
Answer ALL questions.
9. (a) Explain the factor's influencing absorption wavelength.
(b) Calculate the "max values for the following reactions.
|
(ii) | 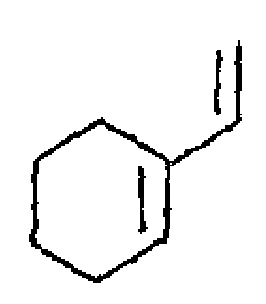 |
|
(i) | 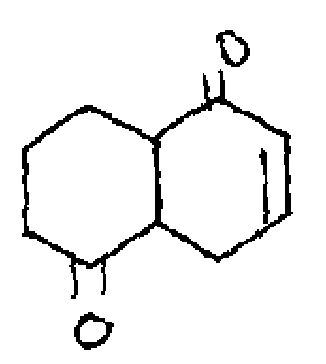 |
Or
(10)
(c) How to distinguish
(i) 1, 2 and 3 amines.
(ii) Cis-Stilbene and Trans-Stilbene by using IR spectroscopy.
(d) Comparsion between intra and inter hydrogen bonding by using IR
(5)
spectroscopy.
Explain Factor's influencing on processional-frequencies of Nucles.
10. (a)
(10)
(b) Give the structure by using H'NMR data : (5)
# 1.3 &3H, t J = 7 Hz); # 3.85 &3H, S)
# 4.3 &2H, q J = 7 Hz); # 6.9 &2H, d J = 11 Hz)
# 7.9 &2H, d J = 11 Hz);
Or
(c) Explain mass instrumentation with diagram. (10)
(d) Tropelium cation in mass analizer decomposed into cyclopenta dienoyl
cation and acetylene give the mass of the meta stable ion in the
mass
11. (a) Write the photo-cyclo addition reaction give suitable examples. (10)
(b) Write the Zimmermann free radical rearrangement of
Or
(c) Explain 4n system by using FMO's approach. (10)
(d) Write the selection rules for PMO's approach. (5)
12. (a) Explain Bio synthesis of Terpenoids. (15)
Or
M.Sc. (Final) DEGREE EXAMINATION DECEMBER 2007.
Second Year
Chemistry
Paper VIII ENVIRONMENTAL CHEMISTRY Time : Three hours Maximum : 100
marks
PART A (4 x 10 = 40 marks)
Answer any FOUR of the following.
1. What is Lithosphere? Describe the principles of weathering.
2. What are the alkali salts present in soils? Explain their analysis in detail.
3. What are the organic pollutants in air? Explain how their limits are minimized to
permissible levels.
4. Explain the radioactive pollutants in the air.
5. Explain the formation of acid rains and their control.
6. Explain the thermal pollutants present in air and their control.
7. Describe the determination of dissolved oxygen and BOD in water.
8. Describe the disinfection methods used for drinking water.
PART B (4 x 15 = 60 marks)
Answer All the FOUR of the following.
9. (a) Explain the functions of soils. Discuss the factors affecting soil development.
Or
(b) Explain the steps involved in the collection of soil samples for analysis.
Describe the determination of humus and total nitrogen content present
in soil.
10. (a) Describe the sources of air pollutants in the air and write the acceptable limits. Explain how hydrogen sulfide is analysed in the air samples.
Or
(b) Write a detailed note on the emission of hydrocarbons and their control in Automobiles.
11. (a) Explain the treatment of thermal and solid wastes.
Or
(b) Describe the water quality parameters for domestic water. Explain the diseases caused by the water pollutants.
12. (a) What type of suspended particulate matter as pollutants in the water? Explain how they are analyzed.
Or
(b) Describe the techniques involved in the water treatment. Add a note on the principle of electro dialysis.
How do you determine the composition of a complex by Electronic
absorption spectroscopy?
Or
|
Attachment: |
| Earning: Approval pending. |
