Indira Gandhi National Open University (IGNOU) 2005 B.Sc Chemistry Organic - - Question Paper
CHE-5
BACHELOR OF SCIENCE (B.Sc.) Term-End Examination December, 2005
CHEMISTRY CHE-5 : ORGANIC CHEMISTRY
Time : 2 hours Maximum Marks : 50
Note : Attempt all the four questions.
1. (a) Give the IUPAC names of any two of the following : 1+1
ch3
I
0) CH3 CH = CH C C = C CHg
I
ch3
(ii) CHgO <>CHO
Br
O
II
(iii) CH. CH, c CHr, CH = CH CH, C OH
II .
CH2
(b) Write the structural formula of any two of the following : 1+1
(i) 2-ch1oro-4-hydroxypentanoyl chloride
(ii) 2,2,4-trimethyl pentane
(iii) 2-methylhept-6-en-3-ynamide
(c) Identify the chromophore(s) present in the following compound : 1
CH. CH = CH C OH
ii
O
2. Attempt any five questions from the following :
(a) Answer any two from the following : 2
O
CH, CH,
CH
ch3 CH - ch2 Wim* , ?
3 2 Na2C03(aq.) (il) Na2C03 (aq.)
(b) Arrange the following in the increasing order of their acid strength :
Fluoroethanoic acid, ethanoic acid and methoxy ethanoic acid
Give reason in support of your answer. 2
(c) Assign R or S configuration to D (+) glyceraldehyde.
(d) Which one is more stable a tertiary carbocation or a primary carbocation ? Explain using hyperconjugation. ' 2
(e) Assign the configuration as or Z to the following compounds : 2
H . CH3
(i) /C = CC
Br
Cl . . COOH
(ii) /C = C(
Br CH3
(0 What happens when glycol is treated with 2
(i) Na at 327 K ?
(ii) Na at 423 K ?
(g) Define iodine value and saponification value. 2
3. Attempt any five of the following questions :
(a) Define chemical shift. Using I.R. and N.M.R. spectral
techniques, differentiate between : 3
(oC-CHa and <-CH2CH3
O
(i) 4-chloropyridine is treated with ammonia ?
(ii) Pyrrole is treated with acetic anhydride ?
(iii) Primary amine is reacted with chloroform in presence of alkali ?
(c) Give one example of each of the following reactions : 3
(i) Oppenauer oxidation
(ii) Wittig reaction
(iii) Gattermann - Koch synthesis
(d) Give the reaction of 3
(i) chloroform with silver powder.
(ii) chloromethane with sodium metal.
(iii) 1-bromo-l-butene with HBr in the presence of
peroxide.
(e) Give one example for each of the following categories
3
of compounds :
(i) disaccharide
(ii) antibiotic
(iii) alkaloid
(f) Electrophilic substitution in pyridine takes place at
3
3-position. Explain.
Explain giving reason.
Attempt any five of the following :
(g) The nitration of nitrobenzene yields m-dinitrobenzene as major product as shown below :
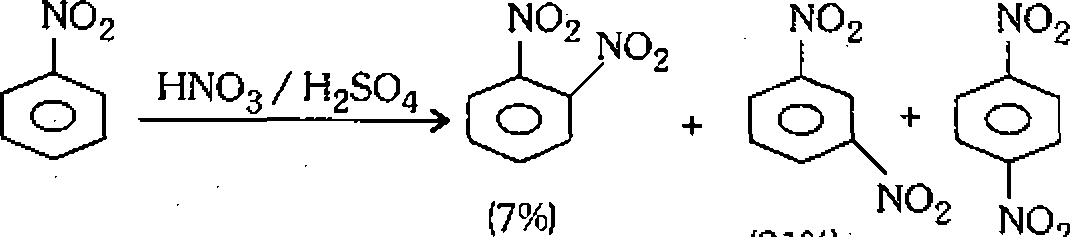 |
|
(91%) 2 (2%) |
(a) An organic compound A reacts with soda-amide followed by methyl iodide and produced B. B on treatment with 40% H2S04 in the presence of mercuric ions yields C. On treatment with iodine and alkali, C yields iodoform and a carboxylic acid D whose molecular mass is 60. Identify A, B, C and D.
(b) An organic compound A on ozonolysis gives two products B and C. Both B and C give positive Tollens test. B gives iodoform and carboxylic acid D when treated with iodine and alkali. Acid D is the oxidation product of compound C. Acid D gives Tollens test. Identify A, B, C and D.
{c) Give one example of the following reactions :
(i) Perkin reaction
(ii) Benzoin condensation
(iii) Riemer - Tiemann reaction
(iv) Williamson synthesis
differentiated with the help of Hinsberg reagent ? 4
(e) What is Michael addition ? How will you synthesize
5-oxohexanoic acid using it ?
4
4
(f) Attempt any two of the following :
Explain the mechanism of
(i) Birch reduction
(ii) Kolbes electrolytic method for the preparation of alkenes
(iii) Aldol condensation
(g) Give the products of the following reactions (attempt
any fouj) : 4
ch2ch3
 |
CrP3 373 K |
40% H2S04
(i) CO,
(ii) CHgMgBr
Ih)h+/h2o>
(iii) CH3COOH + (CH3)2NH 428 K>
{i) LiAlH4 , ether
(iv) CH3COOH
(ii) H+/H20 *
|
- + SO3H | |
|
(v) |  |
CH3
CHE-5 6
|
Attachment: |
| Earning: Approval pending. |
