Indira Gandhi National Open University (IGNOU) 2005 B.Sc Chemistry SpectroScopy - - Question Paper
CHE-10
BACHELOR OF SCIENCE (B.Sc.) Term-End Examination June, 2005
CHE-10 : SPECTROSCOPY
Maximum Marks : 50
Time: 2 hours
Note : Attempt any five questions. All questions carry equal marks,
1. (a) Explain the vector nature of orbital angular momentum {/}. Draw the diagram showing components of anguJar momentum corresponding to
-1-1.
5
3
(b) Compare the number and type of axes of symmetry
present in NH3 and BF3 molecules.
(c) Write the expression relating kinetic energy and
moment of inertia. Also, give the significance of various terms appearing in it.
2, (a) State the meaning of the term, group frequency.
Explain the use of IR spectra in structure
determination using any two examples.
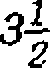
(b) State Hookes law. Also write its mathematical form.
(c) Explain any two of the following terms ;
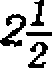
2x2
(i) Zero point energy
(ii) Fundamental transitions and overtones
(iii) P and R branches
3. (a) Draw diagrams illustrating the stretching and bending
vibrations of CH2 group.
3
3
2
2
5
3
(b) Calculate the stretching and bending modes in CH molecule.
(c) Explain the terms, Stokes tines and anti-Stokes lines.
(d) In the S-bvanch of rotational Raman spectrum of C02 (g), a series of absorption peaks is separated by 3*16 cm-1. Calculate the value of rotational constant, B for C02 molecule,
4. (a) Derive the term symbol for the ground state of
hydrogen molecule,
(b) Explain why an aqueous solution of (SOg is red purple in colour.
(c) Name one source of radiation each for recording spectrum in 1R and UV regions.
(b) Define chemical shift. State the name and structure of the standard reference compound used for measuring chemical shift. Give the names of two factors that
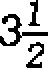
affect chemical shift.
(c) Explain why
2+3
(1) ESR spectra are recorded as derivative spectra, {ii} ESR spectrum of SO shows a single line.
6- (a) Write short notes on the following :
4
(i) Base peak
(ii) a-cleavage
(b) An organic compound having molecular formula C4HgO showed the following spectral data :
Mass spectrum : mfz 72t 43 and 57
IR spectrum : 1716, 1460 and 2941-2857 cm-1
UV spectrum : 274 nm
1H - NMR spectrum ; (5, CDCy :
l'O (3H, triplet),
2-47 (2H, quartet) and 2-20 (3H, singlet)
Assign the structure of this compound on the basis of the above spectral data. Also correlate various signals
6
to the structural units present in the molecule.
7. (a) N20 anc 2 are th molecules. The bands occur at the same wave number in the IR and Raman spectra of N20 whereas it is not observed for C02* Justify these results drawing their skeletal structure.
{b) Define the term, polarisability of a molecule. State the requirement for Raman band in terms of polarisability.
(c) State Franck - Condon principle. Using a diagram, explain any one of the following :
(i) Predissociation
{ii} Phosphorescence
CHE-10 4
|
Attachment: |
| Earning: Approval pending. |
