West Bengal Institute of Technology (WBIT) 2009 B.Tech Computer Science and Engineering Engineering chemistry - Question Paper
. . ,, SEMESTER - 2 Time: 3 Hours . Full Marks : 70
' Group A
(Multiple Choice Type Qaestions)
1. Choose the correct alternatives for any ten of the following : .
- . 10x1=10
(i) van der Waals type of bbhd & fctted?By
(a) sharing of electrons (b) transferring of electrons from one atom to the other t ; (o) sharing of electrons by one atom only (d) , weak electrostatic force of attraction *.<!: ongfluptvpitiijdipples.s , .
(ii) Which of the following defects arises due to misplace of ions in a crystal lattice? -(a) Schottky defect (b) Frenkel defect (c) Metal excess defect (d) Non-stoichiometric defect. : _ \ ,. : ' ' ; ; /
(iii) I he coupling between base units of DNA is through (a) Covalent bonding (b) Electrovalent bonding (c) van der Waals forces (d) Hydrogen bonding.
(iv) C02 is isostructural with . >..= .r r ~
(a) C2H2 (b) HgCl2 (c) N02 (4) NH3
(v) ZnO is white when cold and yellow when hot, this is due to ,
(a) charge transfer (b) d-d transition (c) metal excess defect (d) none of these
(vi) The phenomenon of superconductivity was coined by
(a) Carnot (b) C.V. Raman (c) Einstein (d) Kammerlingh Onnes
(vii) The presence of uitermolecular and intramolecular hydrogen bonding is distinguished
- hy - - - ' v. : . ... . : . . ..
. (a) UV-Visible spectroscopy . (b) IR-spectroscopy
(c) 1 H-NMR spectroscopy , (d) Both IRand 1 H-NMR spectroscopy.
(viii) Caprolactum is a monomer of ;
' (a) Bakeltte (b) PVC (c) Nylon-66 (d) Teflon .
(ix) The ion conductance of an ion depends on its
(a) charge only r. ,. (b) speed only
(e) charge and speed (d) charge, speed and hydration
(x) Anomalous expansion of water from 0-4C occurs due to
. (a) van der Waals interaction (b) hydrogen bonding .
*: ,?(.f(n-<vaJe9]t.5yje8|c interaction(d) <jipqle-induced dipole interaction ? <-xi) ;Tlwhybridisation of XeiniXeF2is t ,, .
(a) sp (b) sp3 d2 (c) sp3 (d) sp3d. , t ,
(xii) [Co (NH3)5 CN] C|2 and [Co (NHj)s NC] CI2 are
(a) coordination isomers ' (b) geometrical isomers
' (c) linkage isomers (d) ionization isomers. T
Ans. Q.l. (i) d ; (ii) a/d.; (iii) d ; (iv) a ; (V) c ; (vi) d ; (vii) b ; (viii) nylon 6 (AH the
options are incorrect) ; (ix) d ; (x) b ; (xi) d ; (xii) c.
Group - B (Short Answer Type Questions)
Answer any three of the following; 3 * 5 = 15
2. Define ionic mobility. Mention the unit Of equivalence conductance and ionic mobility. How does equivalence conductance vary with concentration for both strong and weak electrolytes? 1+1+3
Ans. Ionic mobility : The velocity with which an ion moves under a potential gradient of
1 volt/m. or 1 volt/cm in a solution is called absolute ionic velocity or ionic mobility.
velocity
. Ionic mobility = vo,tage/distance
Unit of Equivalent conductance : Ohm-1 cm2 gm-eq-1 (C.GS.)
Ohm-1 m2 gmHeq-1 (S.I.)
& Ionic mobility : cm2 s_t v~* (C.GS.)
J -\ -I '
m- s 1v 1 . ,
Variation of equivalent conductance with concentration for strong & weak
electrolytes : The equivalent conductance or the molar conductance (Am) of an electrolytes increases with decrease in concentration of electrolyte or with increase in its dilution. Equivalent conductance of strong electrolytes like He I, kcl, CaCl2 CH2 WoN -etc with concentration have a comparatively small in crease through they have very high equivalent conductance at or binary temperature ; where as weak electrolyte, like CH COOH show marked increase* in equivalent conductance at high dilution.
This is due to the result of an inter ionic attraction between (+)ve and (-) ve ions. The two opposite ions may even give rise to some ion pair of the type (A+B). This type of interionic attraction effectively reduces the speed of the ions and ultimately reduce the equivalent conductance of the electrolytic solution. But when concentration is lowered by increasing the dilution, the ioris go far apart, the interionic forces are reduced, Then the iorts can move freely. So in that case conductance can be increased with delution.
3. Write down the possible products on the dehydration of neopentryl alcohol. Write down the main features of transition state theory. What is the unit of rate constant of a second order reaction? 2+2+1
Ana. Possible products on the dehy d ration of neo pentyl alcohol:
CH3 CH3 CHj
* + I 7 - ' 1
ch3-c- ch2oh-> ch3- c - ch2- oh2 ch3- c - ch2
CW3 CHj CHj
2 methyl but-2-ene neopentyl alchol / CH,-C = CH-CH3
" 7 C1I
Lni
1,2 methyl shift 2 3
II N ch2 =c-ch2-ch3
" <H- ck
2 methyl but*l*ene Main features of transition sate theory :
(i) The seacting molecular, possesion necessary energy approach to form an activated complex by rearrangement of atoms.
(ii) The energy necessary to approach the seactant molecular to form this transition state or . activated complex is the energy of activation. -
(iii) For normal or direct reactions, the activated complex formed in assumed to be in equilibrium with reactant.
(iv) The activated complex is not a stable species or a reaction intermediate. It is very short lined. .
(v) The potential energy of the activated complex is very high compared to that of the reactant as well as product.
(vi) Transient state does not represent an oberservable, but can be assumed to possess properties such as bond length, molecular weight, enthalpy, etc.
(vii) The activated complex subsequently break up in to products. .
The unit of rate constant if a second order reaction is lite mole-1 sec-1.
4. (a) What is pseudo-unimolecuiar miction ? Give one example.
(b) Explain the physical significance of activation energy. . 3+2
Ans. (a) Pseudo-Unimolecular reaction : Those reactions which are bimolecular but are of 1st order are called pseudo unimolecular reaction example : Hydrolysis of ester in presence of mineral acid. Hydrolysis of esters in presence of mineral acids follows first order kineticts. eg. hydrolysis of ethyl acctate in presence of HC1.
ch3 COO c2h5 + H20 HC1 ch3cooh + c2h5oh
Here HC1 act as a catalyet and does not take part in the rate equation. The reaction is bimolecular. However the rate of reaction in given by the rate.
rate =\1C [ester] ,
This is because the water is present in large exces and its active mass remain practically constant. Therefore its active mass gets included in tber constant. Since the rate of the reaction
WBUT (2nd Sem)30
h determined by one concentration term only ie ester, the rection is 1st order. So such reaction is called pseudo-tini molecular reaction.
Ans. (b) A chemical seaction occurs as aresult of collision between reacting molecules possessing sufficient high energy to react. Molecules possessing energy less than threshold energy, simply collide and rebonid without reacting. The extra energy which must be given to molecular to enable them to bring about effective collisions is called activation energy (E ). Arcactron whose actuation energy is high will prcecd at slowrate and areaction whose I value is Inn will proceed at high rate. a
|
Potential energy | 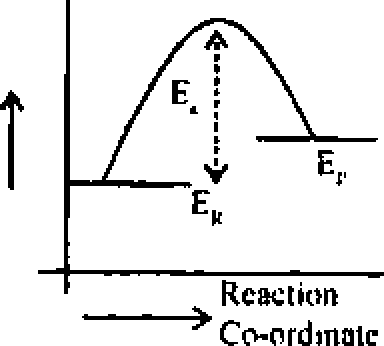 |
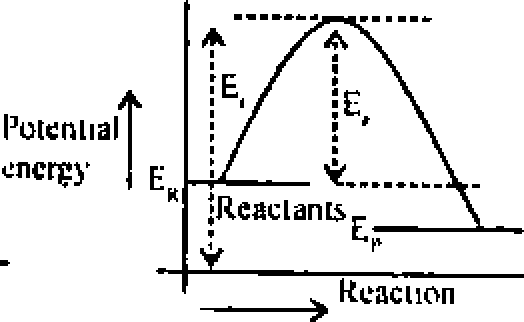 |
A cm alcd Complen aH Product |
|
Coord male | |
when -> ER > Ep. the reaction is cxolhcrmill -
5. Define lattice energy and hybridization. Arrange the following ionic crystals in order of their increasing latticc energy : LiF, CaF2 and Mg S. 3+2
Ans. Lattice Energy : The lattice energy of an ionic compound is defined as the amount ot energy released when oppositely charged ions get packed closely to form one mole of crystalline solid, eg,
NaT + Cl' -+ Nad + 787 kJ.
Higher lattice energy means greater stability of ionic compound and greater attarction between the cations and anions.
Hybridization : Hybridization is the concept of intermixing of the orbitals of an atom having nearly the same energy to give exactly equivalent orbitals w'ith same energy, identical shapes and sy mmetrical orientations in space, eg. Sp hybridization is formed bv mixing of S &
I1 orbital of an atom, '
The order of the following ionic crystal in order of their in creasing latticc enerev is-
LiF < CaF, < MgS
W hat is LPG ? Why is it used as a domestic fuel ? Define Octance number of a fuel and explain how ihe Octance number can be improved ? 1+1+3-Ans.: Q.6. Same as Q.6, 2006.
6.
Group - C (Long Answer Type Questions)
Answer any three of the following.
3 * 15 =45
7. (a) Distinguish between SNj and SN2 mechanisms with suitable examples. Discuss the
role of solvents in SN, reactions.
4+2
(b) State and explain Hesss law with examples. How does it follow the first law of thermodynamics? 2+2
'(c) Using Carnot cycle, prove that the efficiency of a heat engine is always less than one. 6+4+5
Ans. (a) SN1 follow first order kinetics ie they are unimlecular. '
, SN2 follows 2nd order kinetics ie they are bimolecular.
SN1 onechanism is generally followed by testiary halides, eg. alkaline hydrolyisis Of testiary butyl bromide.
KOH K++OH-
(aq) (Nu)
CH, CH3 CH3
First step : CH-C-Br CHi-C+Pr>CHj-C-OH
| r/d step | |
ch3 ch3 ch3 .
tert. butyl bromide tert. butyl bromide
SN2 mechanism is generally followed by primary halidex. eg. alkaline hydrolysis of ethyl bromide and is a one step onechanism.
|-j H
CM-Br-[HOs~ BrS'l HO-C-H-Br0
I L I J l
. (11 Br CH
SN1 mechanism are favoured by weak nucleophiles whereas SN2 mechanism are favaured by strong mucleophiles in the above example OH-.
SN1 mechanism are favoured by polar solvents and SN2 mechanism are favoured by polar aprolic solvents.
Both, back side and front side attack of nudeophile takes place in SN1 reaction. In case of optical active substrate, configuration dominates because of back side attack till the formation of canbocation.
The nucleophile ex-clusively attacks from back side of substrate in SN2 reaction. If the substrate in optically active inversion of config takes place.
The electronic factories influence the reaction rates largely in SN1 mechanism. For the same halogen, the reactisity sequence is tert. > sec. > primary ? CH3x.
Stenic factors influence the rate if reaction in SN2 mechanism. The electronic factors do not influence the rate. Thereactivity sequence is CH3> Primary > Sec.> tert.
Here transition state (T.S.) being more polar man than that of stanting material. This is because, new change is developed and concentrated in the Transition state I (T.S.I) as compared to the stanting material. So in solvents of high polarity, T.S. gets greater stability that of the starting material. As a result, the energy of activation value is decreased and consequently the reaction rate is increased. Moreover, the reaction, rate increases with increasing the polarity of solvent, because the stability if carbocation intermediate increases with increasing the polarity of solvent. Here usually polar profit solvent is used to separate leaving group from carbocation (R+) is a solvation through inteamoleculair H-bonding between X- and H-atom of the solvent molecule. Some example of polar solvent. HjO, RoH, R COOH, R CON2 etc. '
Ans. (b) Hesss law : Same as Q. 10(c) 2007. .
Hesss law follows 1st law of thermodynamics : From 1st law of thermodynamics we know, AE = q-v\ - q-PAV
AE is a state function and its value depend, only on the initial and final states of the system but not on the path of the transformation. However, the values of a and w depend on the path by which the change is carried out
At constant \olume, Av=0, so w = 0 .\ AE-q
Since AE is a definite quantity, therefore at constant volume, q is also a definite quantity. At constant pressure the work done w becomes a definite quentity. Thus at constant prossure, q = AE + PAv. becomes a definite quantity. So at constant volume or pressure, the heat evolved or absorbed in the reaction must therefore, Hens law is only special case of 1st law of thermodynamics. '
Ans. (c) Proof : The efficiency of a heat engine is always less than one using cannot cycle :
When q = 0, ie no hert is rejected to the sink.
W = q-q,
T-Ti , q, Tt a. T, Ti
* q-qrq-T1 or, l-~:il- = i-L or, = or, q = qi
1 T q T q T T
Therefore Wmax =qxT T( xTx =r(T ~ Ti) = 0 ie no work can obtained.
T I) T 1|
Hence to have work, heat must be supplied to the system from the source and more over
some heat must be rejected to the sink by the system ie heat flow fromcource to sink. So
when system do some work, some amount of heat (qj) must be rejected to the sink.
. . q - q j must be < q
w< q r| (efficiency of a heat enjine) < 1
5l<l
a ' ' ' ' .
1 ie 100% conversion is not feasible. Thus only a fractional quantity of supplied heat can be
converted to work and this fraction is,5-.
8. (a) What is fingerprint region ? Why is methanol a good solvent for UV but not for ER. determination ? What solvents are generally used for IR technique ? Which groups are detected if absorption data are 2841(w), 2755(w), 1686(s), 1605, 1460(m) cm-1 cm-1 ?
(b) What is synthetic metal ? What is electronic polymer ? Write notes on conducting polymers and their importance. I ' 9+6
Ans. (a) Finger print Region : -The region 1300 to 625 cm-1 known as fringer print region. It is here that the pattern'of peaks varies from compound to compound. These are some substancees containing the same functional group show similar absorption above 1500 cm-1. However absorption positions differ in fingerprint region. This region is very useful in identifying an unknown compound by comparing its IR spectrum will a set of standard
spectrum recorded under indentical conditions.
' . . . ' ' '
UV spectra are recorded by dissolving the compounds in suitable solvents. A good solvent should be transparent over the desired- range of coavelengths. Usually solvent, which do not contain conjugated system are most suitable for running the UV spectrum. Methanol is a good solvent for UV because it is obtained as very high grade purity and it is free from UV absgrbing substances. . ' ' -
In case if IR spectra, method is not used as solvent because it is not transparent in the IR range and it has strong IR absorption band. It not only dissolve oni the cells made of rock salt but would also give overlapping bands in certain cases.
The most convenient way to obtain IR spectra of solid substance is to use them in solution form. Effective solvent are those which have poor absorption of their own. No one solvent is transparent in the entire region and the solvent lias to be selected keeping in view the region where the compound under study is expected to absorb. The most commonly used solvents in IR spectra scopy are CC14, CHC13 and .CS2.
Cm"1
2841 (W) Aliphatic C-H, Aldehyde C-H.
2755 (W) Aldehyde C-H
1686 (S) Amide, > C = 0
1605 f Aromatic
1460 (m) Ester (aromatic) C = 0, Amine C = ;N
Ans. (b) Synthetic Metal : Polymer that possess the characteristic properties of metals , such as electrical conductivity while retainting the mechanical properties processibility, etc commonly associated-with conventional polymers are called intvinsically conducting polymers (1CP) or synthelic metal. Example of syathetic metal is the crystalline poly sulphubmitride polymer (SN)n. It has an appreciable conductivity at room temperature.
Electrinic Polymer : Semiconducting polymers whose conductivity can be increased by several times are generally known as electronic polymers. The conductivity of electronic polymers can be increased by doping.
Conducting polymers and their importance : In general polymers are poor conductors of electricity while metals are good conductors. There are some organic polymers such as poly aniline, poly pyoole, poly this phene, etc. They are good conductors like metals. These polymer compound are wholly composed of elements like carbon, hydrogen and occasiowally nitrogen, oxygen or sulphur. More over they have the mechanical properties of polymers, such as flexibility. Such type of polymers are called conducting by a zieglat-Natta process is a
conditctong polymer because the conjugated double bonds in polvacetylene make it possible to conduct electicity down its back bone.
Zi*lar-N.ua_cH = CH[CH = CH]nCH = CH_
HC = CH-Aeetvlene
eatalyst
Polyacctylene
There are some non conducting polymers can be made conducting by doping will an electron dnar or electron aceptor. Thus non conducting polymers are a physical mixture of non conducting; polymers and doping materials. Both n-type and p-type dopants can be used to transform an electronic polymer from an insulator to conductor.
Most of the characteristic shown by inorganic sensiconductors are also shown by semiconducting polymers and the high performance devise's suph as photovoltic cells, photodelectors. 4ightemining diodes, etc made from these polymers have been found to perform to the same levels or even better man the devices made from inorganic semiconductors conducting polymer are such lighter than normal metals and can be'used form making light weight batteries. One & their greater flexibility, the electronic devices such as transistors, made from them can be bended like plastics. '
9. (;i) What are rectifiers? Where are they used?
< b) Why ZnO is white but changes colour on heating ?
(c) W hat are photovoltaic cells?
(d) What is the effect of temperature on the conductivity of p-type and n-type semiconductors? 4+3+4-M
Aiis. (a) Same as. Q. 8(b) 2002.
Ans. (b) Same as Q. 3(c) 2002.
Ans. (c) Photovoltaic Cells : Photovoltaic Cells in a device that directly converts sunlight to electicity.
The most commonly used photovoltaic cells are of the bassier type like iron-selenium
cells or Cu - CuO, cells. In the iron selenium cell, a selenium layer is placed on iron disc.
Now an extreml; thin transparent layer of gold or silver is deposited on the selenium to act as
a front electrode. A contact ring on the silver Light _
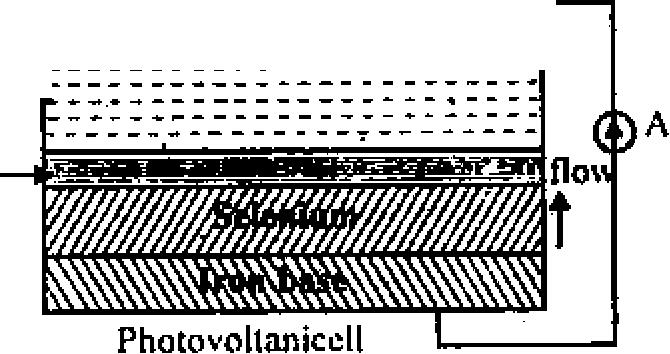
layer acts as one electrode and the iron base as ///// rhRin8|
- Transparent r-----------------------
the other. r.. 1 *
Film
When light falls on the semiconductor. ieBassicr selenium, it ejects electrons which travel fromlaj'er selenium to the front silver electrode through the bassier layer. The flow of electrons in the opposite direction is not permitted by the bassier
because it act as a rectifier. The e.m.f generated internally between silver electrode and selenium is directly proportional to the intensity of incident light radiations.
It inquires no extern al battery for its own operations, ie it is self generating.
The internal emf and hence current generated by it are large enough to be measured on a pointer galvanometer Hence, such cells aie used in devices like portable expouse meters, direct reading illumination meters and low resistance relays for on/off operations and other monitoring operations in industry.
Ans. (d) Effect of temperature on the Conductivity :
Conductivity of n-type and p-tvpe semiconductors increases with increase in temperature. In case ot conductors the free clectom concentration and hole depends temperature.
n - type Semiconductors : For loop n-type semiconductors close to absolute zero, there
are no free carriers in the conduction band, as was in the case of-----semiconductors. Hcnce
the conductivity is negligible. With increase in temperature, the conductivity increases bin
alter a particular temperature it become constant and then again increases with increase in temperature.,
P - type semiconductors : Also for p-type semiconductors, the conductivity increases with increase in temperature, reacting a maximum value and then it becomes constant as in case of n-type semiconductors. However, the increase in conductivity with increase in temperature is mainly due to the increase in number of electrons front the values band of the semiconductors to the accptor band if the doping agent. When all the acceptor alom get filled up will the electrons from the value band, conductivity becomes constant. Now above this
temperature, ihe rise in conductivity of the semi conductor is mainly due to the increase in___
- conductivity.
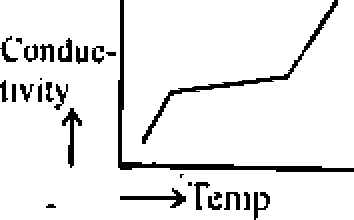
Effect of temp erature on conductivity of type terms conductor.
10. (a) Phosphorous forms both PF3 and PFS but nitrogen forms only NF3. Exnlain.
(b) Arrange the following in increasing order of bond lengths : 02, Oj-.
(c) Using Kohlrauschs law how do we determine the equivalent conductance of acetic acid at infinite dilution?
(d) Carboxylic acid is stronger acid than phenol.* Explain.
(e) What is calorific value of fuel ? Distinguish between gross and net calorific
values.
(0 What arc the constituents of coal as determined by proximate analysis?
Am. (a) The ground state electronic: configuration of phospHorous is-
3s 3p 3d
|
3d | |||||
|
3d
e.s. :
3p
? I? I?
Here phosphorous has vacant 3d orbital. In excited state phosphorous may promote an electron from 3s to vacant ed-orbital. It PF5 phosphorous having gained five electrons from fluorine alons and through sp3d hybridization phosopherous formed PFj. It can also form PF3
by Sp3 d hybridization phosphorous having gained three electrons from---atoms in PF3
molecule.
The ground state electronic of nitrogen is
2s 2p '
it
S,ince nitrogen does not have any accant d-orbital, it can only from NF3 by Sp hybridization.
Ans. (b) O2 => 16 e
106
_2
|
2py n2pz *2py *2pz | ||
|
2Px | 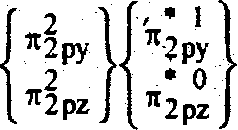 |
B.O. = |
7 *2 ct2S ctIS a
=> 15 e
2, *2 ct2S 2S
IS2 aIS 2
CTIS CTIS
17 e
= 2
B.O.
2Px
10-5
= 2-5
O,
IS CTIS
|
>2Px | 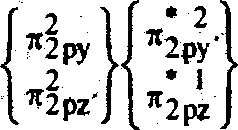 |
B.O. = = 1-5 |
2S 2S
greater the Bond lower the bond length.
So the in creasing order of bond length is o| <02 <03' Ans. (c) Equivalent Conductance of acltic acid at infinite dilution using kohlreushs
law : Equivalent conductance of eveak electrolytes such as CH3COOH, can not found out by extra polution of the curve obtained when exponmentally determined values of equivalent conductance of the electrolyte are plotted against Vconc However kohlraushs law helps to find out Aa for CH3COOH (weak electrolyte) by knowing a 0 values for certain strong electrolytes as CHjCOONa, Hel and Nacl.
Ao(CH3COOH)-%jC00.+>.0h.
|
Adding and subtracting limiting ionic conductances of each of Na* and cl to the R.H.S. w e get. |
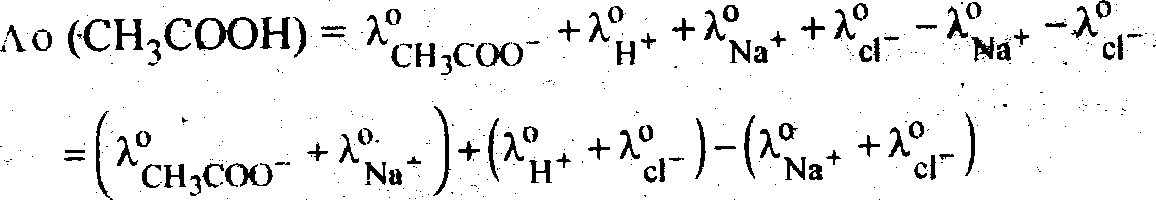 |
Ao (CH3COOH) = A0(CHjCOONa) + A0(Hcl)-A0(NacI)
Hence by knowing A0 for strong electrolytes ie CHCOONa, Hct and Nacl, A0 for CHCOOH can be found out.
Ans. (d) The stability of conjugate bases is in the order
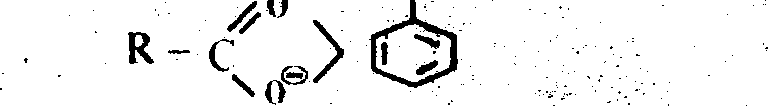
R = alkyl group.
The delocalisation structure ofcarboxylate anion is as follows :
9
R- C ur* R- C
and the phenside ion is
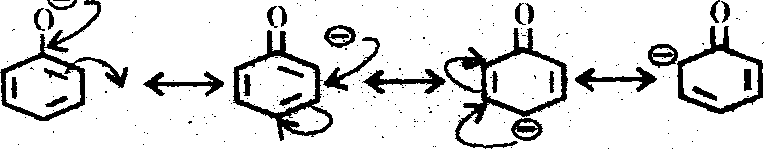
Phenols are considerably weaker acid than carbonytic acid. This is due to the fact that
delocalisation of the (-) ve charge in the carbonylate anion-----structures of indentical energy
content and of the centres of highly electronegative oxygen atom ; where as in the phenoside anion, the structures be higher energy content than the one in which it is on oxygen atom. The relative stabilization of anion w.r. to the undissociated molecule is these likely to be less effective with aohenol than with a carbolytk acid, leading to the lower relative acidity of the former.
Ans. (e) Carbolitic value of fuel: Calorific value of a fuel may be defined as the amount of heat produced by completely burning of unit man or volume of the fuel. Higher the calorific value better the fuel will be. S.I. unit: J kg * and C.G.S unit: caj g *
Distinguish lactocen gross and net calorific value : Same as Q. 9(a) 2003.
Ans. (0 Constituents of coal as determined by pnzimate analyes. : This analysis provides data for a first general assessment of the quality and type of coal. The properties tested include.
(i) The determination of noister content.
(ii) Volatile Carbonaceons Matter (VCM)
(iii) Ash content .
(iv) Fixed carbon
(a) Biodegradable polymers.
(b) Mass spectroscopy and its applications.
(c) Straight run gasoline and-jet fuel.
(d) Calomel electrode.
(e) LDPE and HDPE
(0 Clausius - Clevperon equation. .
OR '
Gibbs - Helmholtz equation. .
Ans. (a) Biodegradable Polymers (out of syllabus) : The polymers that can be broken down rapidly by enzymen catalysed seactions are called bio degradable polymers.
The enzymes are produced by micro-organisms. The carbon carbon bonds of chain growth polymers are insert to enzyme satalysed seactions so they are now biodegradable unless bonds that can be broken by enzymes are inserted into polymer. When the polymer is burried waste, micro-organisms present in the. soil can degrade the polymer. One method of making a polymer biodegradable insolves inserting hydrolyzable ester* group into the polymer. For example, if the acctal (shown below) is added to an alkene undergoing free radical polymerzation, ester groups will be inserted into the polymer. ,
. /HKcn, vjjo'cii.'vni
CHy-CII-Cll. C lu I,
2 1 ' 11 I " N>-CII( 11
11 - R - - , '
' ' ' O / ' ' ' O
- II . pu_ _ rjjp . . II - . ,
CH.-CH-CH,-C~O-CH.CH.CHXIICHXH < CH, -CH~CH2-C -Q-CH2CH:CH,
I . \ I |
, pi A weak line r . R
The ester link being a weak link is susceptible to enzyme catalysed hydrolysis. Among the most common biodegradable polymers are poly glycolic acid (PGA), poly lactic acid (PLA) and poly hydroxy butyrate (PHB). AH are'polyester and are therefore suceplible towards enzyme catalysed hydrolysis of there ester link. Co-polymers of PGA and PLA hare found wide variety of.uses. For example, a 90/10 co-poly glycolis acid with poly laptic acid used to make absorbable sutures (thread used for goining the edges of wound by stiching). Dextron was the first biodegradable sature. She Stares are entirely degraded arid absorbed by the body within 90 days aftpr surgery. Poly hydroxy butyrate which can be used for making films for packaging as well as molded items degrades within fore weaks in land fills. However at present this polymer has limited use due its high cost which is about four times as compared to polypropylene.
Ans. (b) Same as Q.l2(c) 2002.
Applications : (i) Identification of substances : Man spectrum is highly characteristic of a compound. No two compounds can have exactly similar man spectra.
(ii) Determination of molecular man and formula.
(iii) Determination of molecular structure.
Ans. (c) Same as Q,8(c) 2005.
Ans. (d) Calomel Electrode : Calomel electrodes is an example of secondary reference electrode. A calomel electrode consists of mercury in contact with a paste of spasingle soluble salt of mcrcurous chloride in mercury and a solution of soluble chloride ions such as Kcl known concentration. It is represented as :
Cl (aq)/Hg2Cl:(&)/Hg(/)
I'he half cell reaction is
Hg2CU<fc) T 2Hg(/) + 2 Cl" (aq) .
Which is the resultant of the following reactions taking place :
|
noiJulos 0>f p W*. |  |
|
aboimia tomoleO | |
Hg CMs) H;2(aq) + 2CI-(aq)
HgJ:(aq) + 2e" 2Hg (1) '
I he calomel electrode is, therefore, reversible w.r. to Cl ions (anions).
In case a saturated solution of KCI is used, the electrode is called saturated calomel electrode. This electrode is commonly "used in laboratory. A dip type saturated calomel electrode is now available for use.
The electrode potential of electrode on H-scale, represented as
KCI(aq)/Hg3CI2(s)/Hg depends upon the concentration of KCI solution contained in it and on the temperature.
Hg CI2(S) + 2e 2Hg (/) + 2Cr(aq)
The tenth molar electrode (0.1M KCI) has the lowest temperature Co-efficient and is preferred for accurate work. However, saturated calomel electrode is tiie most convenient due to the case of replacing the solution and is, thus frequently employed.
Ans. (e) LDPE : It is manufactured by heating ethylene to 350 K to 570 K under a pressure of 1000 - 2000 atm. and in presence of traces of oxygen (0 03 - 01y.)
350k-570k,02
n CH, - CH2 ]ooo-2000 aim (CH2 CH2> polyethylene
This polymersiation reaction occurs through a free radical mechanism initiated by oxygen. Poly there produced by this process has a molecular mass around 20,000 and has a branched chain structure. Since branched chain molecule do not pack well, this type of polythene has low density (0-92 gm cm-3) and low melting point (384 K). That is why polythene produced bv this method is called low density polythene.
Propcrtis : It is transparent, has moderate tensile strength and high toughness. It is chens cally insert, slightly flexible and poor conductor of electricity.
Uses : It is used (2) as packing material (in the form of this film, bags, etc. ; (ii) for insulating sires and cables, etc.
HDPE : It is manufactured by heating ethylene at about 333 - 343 K under a pressure 96
- 7 atm. in presence of- a catalyst such as trieltyl-aluminium and titeanium tetrachloride (zeigiar-Natta catelyet).
333 - 343K,6 ~ 7atm "
n CH2 - CH2 Seigler_Natta Catalyst (CH2 - CHj) Poly ethylene.
Properties : Polythene produced by this process mainly consists of lineear chains. Therefore, the moleculer pack well and hence this type of poly thene has density (0-97 gm cm 3), higher melting point (313 - 423K) and is inert but harder, tongher and has greater tensile strength than LDPE.
Uses : It is used (i) In the manufacture of container (buckets, tubes, etc.)
(ii) for making pipes, bottles, toys, etc.
(iii) As insulators, anticorrosise and packaging material.
Ans. (f) Same as Q. 10(a) 2003.
OR. Same as Q.3(d) 2005.
|
Attachment: |
| Earning: Approval pending. |
