West Bengal Institute of Technology (WBIT) 2008-2nd Sem B.Tech Computer Science and Engineering Computer Science - Chemistry solved - Question Paper
SEMESTER-2
Time: 3 Hours] I Full Marks: 70
GROUP-A ( Multiple Choice Type Questions ) . .
1.Choose the correct alternatives for any ten of the following : 10 x 1 = 10
i) A living system is thermodynamically an example of
a) an Isolated system b) a dosed system
-c) an adiabatically closed system d) an open system.
ii) For an ideal gas undergoing free expansion . a) AT = 0 and AS >0 b) AT = 0 and AS = 0
c) AT <0 and AS>0 d) AT < 0 and AS = 0.
iii) Structure of SF6 is 1
a) planar b) octahedral c) trigonal bipyramidal d) square pyramidal.
iv) Two elements, whose electronegativities are 1.2 and 3.0, form
a) ionic bond b) covalentbond c) co-ordinate bond d) metallic bond.
v) Germanium is an example of
a) intrinsic semiconductor b) n-typesemiconductor
c) p-type semiconductor d) insulator.
Vi) Proton NMR is usefiriffor investigating structure of organic molecules because
a) organic molecules contain carbon atoms
b) organic molecules are mostly covalent v
c) hydrogen atoms are found in most of the organic molecules
d) organic cofflouads are low boiling.
vii) The half-life of a first order reaction is 20 minutes. The time required for 75% completion of the reactipn is . .
a) 30 minutes c) 40 minutes b) 50 minutes d) 60 minutes.
viii) The half-life of a ftfpt order reaction is 20 minutes. The time required for 75% completion of the rea&km jfefc
a) H20 )H2S c)PH d) NH,
viii) Which one has the largest bond angle ? . ;
a) H20 PS c)PH . d)NH
ix) Which of the olahedraf complexes ( M = metal atom, A and B are ligands ) exhibits geometrical isomeriste ? ,
a) [MA6] b) [MASB] c) [MA4B2] d) [MA3B3] .
; 448
*
WBUT (ENGINEERING CHEMISTRY) QUESTIONS2008
x) The most stable carbonium ion is .
c) CH3CH*
c) CH =CH -CH +.
a)(CH})2CH" b)PhC
xi) An exam|>te of thermosetting plastic is
a) FVC b) nylon c) polythene d) bakeiite
xii) The calorific value is highest for
a) water gas b) LPG c) producer gas d) carburetted water gas.
Ans.: Q.1. (iXd); (iiKa) ; (iXb); (ivXa); (v*a); (bXc); (viXc); (viiXb); (viiiXd); (ixXcXd.); (xXb) : (xiXd); (xiiXb). GROUP - B
(Short Answer Type Qaestteas)
Answer any three of the foBowing 3x5 = 15
2. Show that for an ideal gas undergoing reversible adiabatic expansion or compression PV / = constant. A diatomic ideal gas (7 * 1.4), initially at 600 K aad 10 atm undergoes reversible adiabatic expansion till the final pressure becomes 2 atm. Find out its final volume; '
Ans. Proof PV? = Constant: Same as Q.2 (b) 2005
Numerical Problem : Number of moles, the final temperature and the initial volume are not given here. Hence the problem cannot be solved on the basis of (Mite assumption.
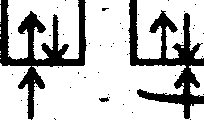
I

| ||||||||||||||||||||||||||||||||||||||||||
|
F~ F F~ F~ Sp3d2 Hybridisation | ||||||||||||||||||||||||||||||||||||||||||
(Six electron pairs from six F~ ligand)
The complex anion [Co(CN )6]-3 has octahedral geometry and due to presence of paired electrons and also involvement of inner d-orbital ie low spin complex, it is diamagnetive.
WBUT (2nd Sem)29
Q.4. Write down the Arrhenius equation for the temperature dependence of specific rate and explain the terms used. What is the unit of the frequency factor for a first order reaction 7 Plot logk vsj and explain the significance of the slope of the plot.
Ans.: Arrhenius equation for the temperature dependence of specific rate : k = AeEa/ RT
A = Arrhenius constant as frequency factor ie frequency of col iision of the reacting
molecules.
R = Universal gas constant , *
! Ea = Activation energy, . .
' ' J : ,
Unit of frequency factor (A) for 1st order reaction :
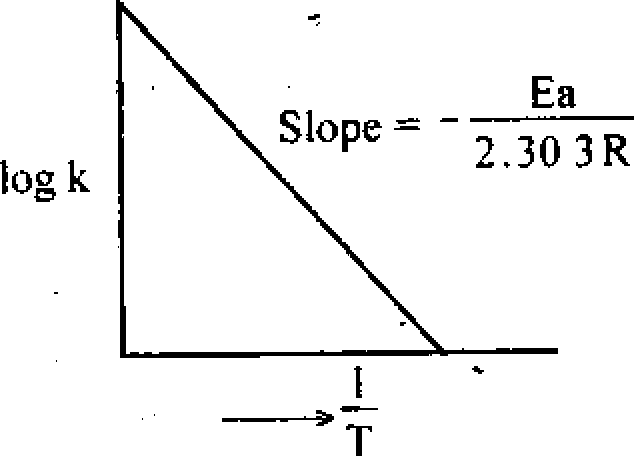
Collision per second t litre Explanation of Plot iog k VS ~
Ink = In (Ae _Ea/RT)
Ea
.* log k = log A -
2.30 3 RT
So if we plot log k against a straight line having a slope equal to Ea / 2.303R and an
intercept log A can be obtained. From the slope we can calculate the activation energy.
Q. 5. Predict the shape of the following molecules using VSEPR theory :
' BF 3, CO 2 i PC/ 5, SF 6, XeF 4 >
ep = 3 plane trainglc bp - 3
|
Ans. BF3 : Central atom B => 3 + 3 = 6 |
 |
= 3-3 = 0
F-B-F= 120
C02 : Central atom C => 4 + 0 = 4
linear
ep - 2 : bp = 2 Ip = 0
linear
PCI5 : Centra! atom P => 5 + 5 = 10 ep - 2 :
Ip = 0 a = axial e = equiatoriai
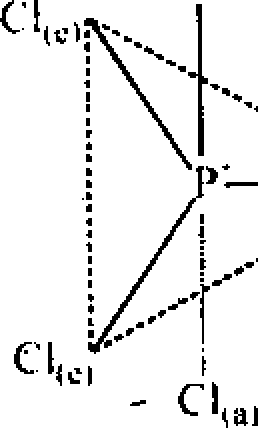
Cl(e)-P-CI(c)-120 CI(o -P-Clw-90 CI(rt -P-Cl(arl80
ta)
SF6 : Central-atom S => 6 + 6 = 12 ep - 6 => Octahedral (Oh) i" bp = 6 Ip = 0
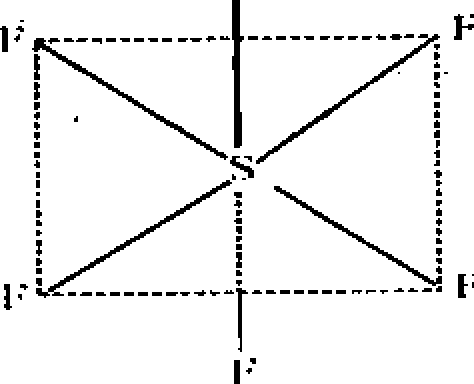
F - S - F * 90
|
Ip 'c |
 |
|
I--------- Ip c |
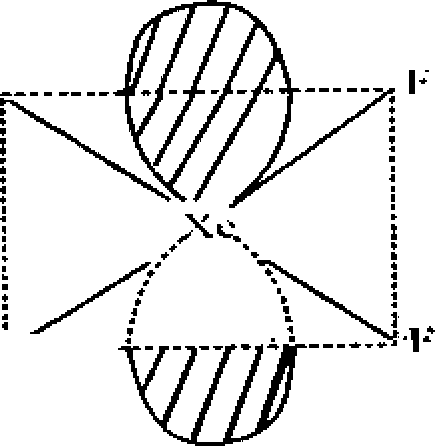
XeF4 : Central atom Xe=?8 + 4= I2=> ep = 6 =>Square plannar K . * bp = 4
Ip = 2
. F - S - F = 90
Account for the following :
Q.6.
i)
H)
SN 2 reaction occurs with inversion of configuration and SN1 reaction occurs with retention of configuration.
Formic acid is more acidic than acetic acid and acetic acid is more acidic than phenol.
Ans. SN 1 : Substitution Nucleophilie bimolecular and 2nd order :
This SN2 mechanism is generally followed by primary alkyl halides (e.g. Methyl bromide). It is a single step reaction. The breaking and making of bond occurs simaltaneously. The nucleophile (e.g. OH-) approaches the carbon atom attached to the halogen (here bromine in example) from the side opposite to that carrying the halogen. This occurs due to the fact that the attack from the side halogen (Br) is hindered, as both nucleophile and halogen (Br) atom are electron rich and cause repulsion. So the product formed has opposite configuration to that of the methyl bromide (like an inverted umbrella). The change in configuration is called the walden Inversion. Then we can say the SN2 reaction
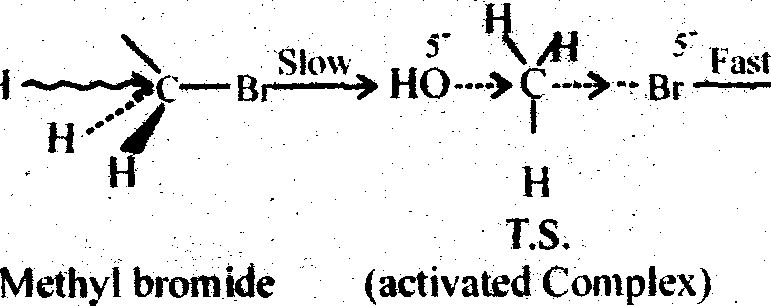
SN 1: Substitution Nucleopbilie Uniaioiecuiar ad 1st order:
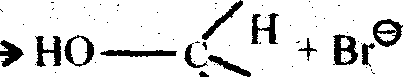 |
|
Methyl alcohol (Inversion of Configuration) H |
This SN1 reaction is a two step process and it is generally followed by tertiary alkayl halide (e.g. Tatiary butyl bromide). Indie 1 st step a carbocation is generated by breaking of carbon-halogen (bromine) bonds. And in the 2nd step that carbonium ion is attakced by a nucleophile ami created the substituted product (Tertiaiy butyl alcohol). The carbonium km formed in the 1st step is plannar as the central positively chased carbon atom is sp2 hybridised. The plannar C+ ion can be attacked by the nucleophile (OH) on either side to give two isomers (d & 1), if thealkyl halide taken be assymetine.
Alkaline hydrolysis of tertiary butyl bromide Via SN1 mechanism
|
+ |
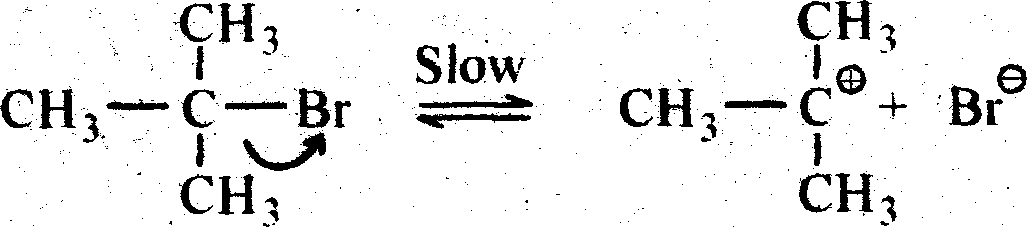 |
KOH (or) s= K OH
Tertiary butyl bromide
|
fast CH, C| + OH > ch3 |
I J  |
|
Tertiarybuty! alcohol | |
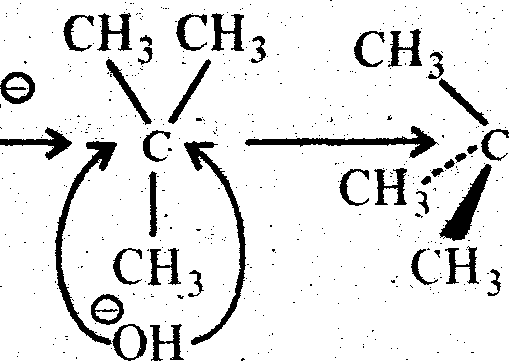 |
OH |
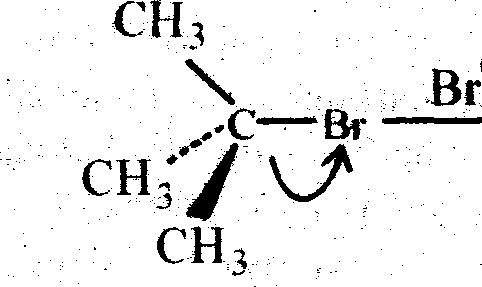
(Retention of Configuration)
*0'
h-c;
|
0 O H * CH,ce + || |
 |
CHHSp
o o
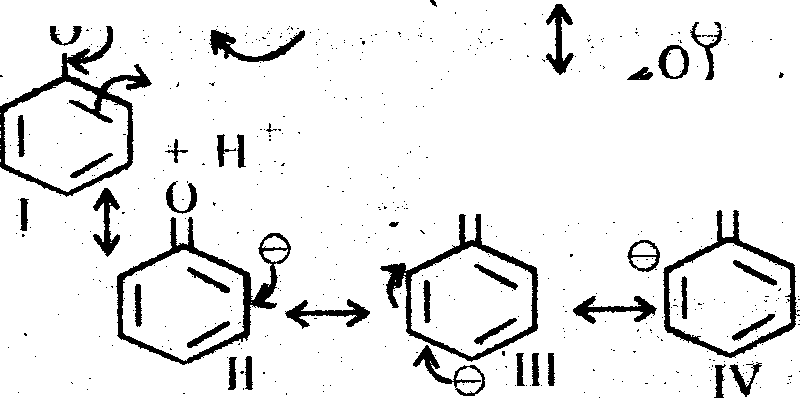
(ii)Formic acid is more acidic than acetic acid because in formic acid no + L effect and no + R effect, conjugate base is m os( stable w here is in ccljc H has U eHecljC H ,3gr) and o + R effect, conjugate base is Jess stable.
Acetic acid is more acidic than phenol (no + I effect and + R effect) Phenoxide ion has four resonating structures but are non equivalent whereas acetate ion although has two equivalent resonating structures and hence more stable than phenoxide ion. .
GROUP-C ( LongAnswerTVpe Questions) Answer any three of the foHowing
3*15*45
7. a) What are raw rubber and vulcanized rubber ? * 3
Ans. Same as Q. 10(a)2002
b) Give the outlines of preparation, structure and uses of SBR and NBR. * Ans. Same as Q. 10(b)-2002
c) Explain number average and weight avenge niotor wass o a polymer. 4 Ans. Namber Average Molecular Weight: Number average molecular weight is determined
by colligative property measurement and it is defined as the total weight of all the molecules present in the polymer sainptfe divided by the total number of molecules present.
Thus if a polymer sample contains nt, n2.............. n* number of molecular species having mas
Mt, M2. M3 respectively, then the numeber ayfeTnqleculwjveigfet If given as:
Total mass of the polymer sample
=
Number of molecular species present in the sample
' XniMi
n1+n2+................+n j 2ni
. - . - i ' - > '
Weight Average Molecular Weight: Weight average molecular weight is generally determined by fight scattering measurement and it is defined as the total contribution of various weight fractions of polymeric species towards the total molecular mass of the polymeric sample. So M w is given
as ; ' . v > -
M7 = W|M,.+ W2M2 +...........+WiMi = WjMi /.
' . ' i -
' Mass of species i *5
where i =-. .
Total Mass of polymer
njM j njM; njMj + h2M2+................ rijMj n;Mj
- - - - j - ,
KC = M, - -- *- - 2+...,............Mi -?1' -
-(n1Mi2.+n2M22 +.................+njMj2 )
niMi ...
1
2
i i
__ i_
J
Wj = fractional weit offcofssponding polffnier unit (i) W = Total weight of the Polymer Sample 1 Mj ~ Molecular mass of thtrPolymerk: Uifiti
Moreover since M v - Mn in ease W monodisperse system'
d) Define intrinsic and extrinsic semiconductors. 4 2
Ans.: Same as Q.3(b) 2002
8. a) What is meant by transport number of an ion ? How is it related with ionic conductance ? The ionic radius of Li'*' is less than that of K +. However, in aqueous solution Li
+ ions are less mobile than K + ions. How can you explain this observation ? I + 1 + 1
Ans.: Transport number of an ion: The fraction of total current carried by a particular type ot ion is known as the transference number or transport nuber of that ion.
If Xv k are ionic conductance and u, u_ are mobility's of cation and anion respectively then the transport number of cation & anion can be written as, _
X+ Fu+ = u+ .
t+ ~ +X_ ~ F<u + +u-) u++u_ k_ Fu_ u_
t_ = X++ f,(u+ +Tj"u++u_
The ionic conductance of Li+ ion is less than of K+ ion in aqueous solution or in other words Li- ion less mobile in aqueous solution than ion, The probable cause in the hvdiation of ions. The Li+ ion being smaller compaired to K+ ion, the electric field in Li is much stronger than K" ion and hence it can capable of associating or attracting large number of water molecules than the K+ ion, The net result is the higher hydration contributing the larger dimensions, lowering the speed and hencc lowering the ionic conductance. The hydration number of Li+ is 6 and K"*" is 2
b) Draw the conductometric titration curve for HC1 vs NH 4 OH and explain the features of the curve.
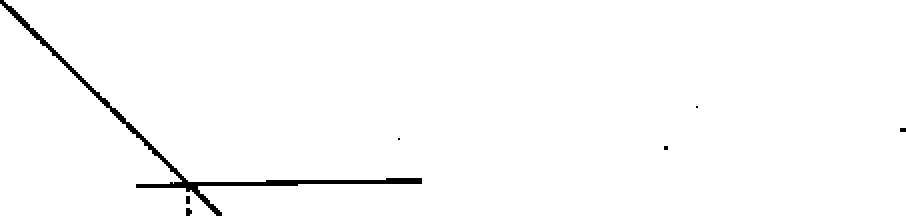
Ans. : Conductance
_j, equivalence point
volume of.NHOH solution
During titration of strong acid (HC1) by a weak base (NH4OH), the following reaction taken place :
H+ + Cl - + NH4OH NH4+ + Cl ' + H20 Here neutralisation reactions means, the replacement of highly conducting H+ ion by low conducting NH4+ ion. So the overall conductance of the solution decreases progressively with the addition pf NH4OH and reaches the lower limit at the equivalence point, after the equivalence point, the conductance of the solution reamain almost constant, as NH4OH, the unused base used further, is a weak base and has a very low ionic conductance.
c) What do you mean by half-decomposition period of a reaction? Consider a second order reaction A + B -> products. Assuming the initial concentrations of both the reactants to be same, show that the half-decomposition period of the reaction is
inversely proportional to the Initial concentration. 1+3
Ans. : Half decomposition period of a reaetioa : For a chemical reaction, the time for half decomposition is called half decomposition period of that chemical reaction. It is denoted
by t, '
Let us consider a second order reaction and the initial concentration of both reaction are same. Say a mole / lit and at time t x mole / lit of each is decomposed to form products.
A 8 Products (2nd order reaction) at t - 0 a a 0 .
att = t (a-x) (a-x) a = initial concentration
at t = 0
under such conditions, the rate of the above reaction .
= k (a -x)(a-x) dt. .
dx -> dx , ,
or,< = k (a ~ x) = kdt
Now infegrating,
-i = kt + C (integraiton constant)
a - x
when t = 0, x = o ,
Hence, = k + CC = a Now substituting the vahie of C in the above equation,
* = kt + - or, kt = X
a-x a a(a x) 11 . . x
or, . = kt k = :--
a-x a t a(a r- x) x
so the rate constant of the above reaction is k =
t a(a - x)
So we can say the half life period 0l) of a second order reaction, having the reactant same initial concentration is inversely proportional to the initial concentration of any reactant.
d) Rate constants of a reaction at 366 K and 310 K are 4.5 X JOs"1 and 9 x JO-V respectively. Evaluate the activation energy and the pre-exponential factor ( frequency factor ) of the reaction. WHat is the order of the reaction ? 4 As. We know, k = A.e~Ea/RT whose k = rate constant
A = Arrhenius constant as frequency factor R = Universal gas constant '
Ea = Activation Energy taking log on both side
Fa
log k, - log A - kt = '4.5 x 10~5 sec-1 at T, = 300K
2.303 RT2 ,
Ea Ea
Now, log k, - log k2 = ~ 2.303 RT, + 2.303 RT2
-, k2 = 9 x 10~5 sec-1 at T2 - 31 OK
2.303 RT,
Ea
log4.5 - log5+ 2.303x2x300 - A = 9.6659 x lO4
9. a) Explain optical isomerism in case of co-ordination compounds with suitable examples. 4
|
Now, log |
| ||||||||||||
|
i k! _ Ea g k2 2.303x2 | |||||||||||||
Ea - - log (0.5) x 2.303 x 2 x \ Ea = 53.604 U
log A
cal =1.2895 K.Cal
45x10
9xl0~5
Ea
or,
log k2 = log A - ,
310 - 300 300 x 310
10
or,
i-5
log
300 x 310
300 x m 10
12895
2.303 x 2
458 . > WBUT SOLVED BOOK - .
Ans.: Same as Q.7.(c)2002 , _ :
b) Write down the order of the bond energy of the following borids and give reason for your answer : C = 0, C = N, C - I, C - F.
Ans. t to case pf Co the bond order is 3 and for CN the bond order is 2.5. In C-I and C-F, thf tefrtd .present is single bond. We know that the higher the bond order higher will be bond energy So the bond energy of C = 0 is greatqr thai\ ,C = Nt.
Now the bond length of CI is 0.214 nm and C F is 0.132 nm. We know that lower the. bond length greater will be bond energy. So bond energy C-F is greather than C - I.
So the bond energy order C = O > C = N > C - F > C - I.
e) Explain the term hyperconjugation, citing examples. ' * 4
Ans. : Hyper conjugation or (Baker Nathan) effect involves the delocalization of sigma (ct) electrons through the overlapping of p-orbital of double bond with a - orbital of the adjacent single bond. As for example propene :
h h ir ii ii H * * ii
-cc = cL -> HC= GG0 C* CCe-* HC = CV
A H H H hi H }\ H H H1 H H
Propene 1 li
on other hand we can say that : (1) hyper conjugation is also called no bond resonance, since no bond exist between free H+ and C-atom, (ii) the H+ions are not free to move, since they still bound quite firmly to the n could, (iii) the hyper conjugation occurs through H-atom present on a -hydrogen atom is on the C-atom adjacent of double bond, (iv) the higher the number of a-hydrogen atoms, the higher is the hyper conjugative effect. Consequently, the order of hyper conjugative effect is :
CH3-> CH3CH2-> (CH3)2CH-> (CH3)3C-
d) Distinguish between coking coal and caking coal. 3
Ans. : Coals that soften on heating in the absence of air, producing a Pasty or Plastic mass which fuse together to large Coherent masses impeivious to air are called caking Coals. Coals from which very little of such plastic material is formed are either non coking or weakly caking coal. *
On the other hand coals on heating produce residue, which is hard, porous strong and usable for metallurgies purpose are called Coking Coal. All coking coals are Caking coals but all caking coals are not coking coals.
The moisture content of coking coals is about 2% but that of weakly caking and noncaking coals is 3 to 12%
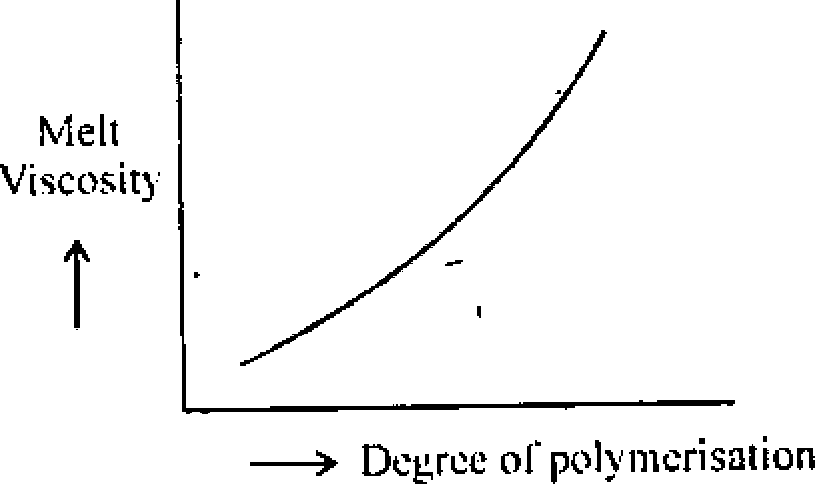
lO.a) How do the properties such sis (i) tensile strength (ii) physical state of the polymer (iii) impact strength (iv) melt viscosity of a polymer vary with degree of polymer-
Ans. : Tensile Strength : Tensile strength of a polymer depends on degree of polymerisation of the polymer. It increases with increase of degree of polymerisation and after a certain tensile strength it stabilises.
Physical state of Polymer : Physical strength of a polymer increases with increase of
degree of polymerisation.
Impact strength of Polymer : impact strength of a polymer increases with increase of
degree of polymerisation.
Mult viscosity of polymer : Melt viscosity of a polymer follow the relation with degree of polymerisation, shown by the graph below :
b) Discuss the difference among isotactic, syndiotactic and atactic polymers. 3 Ans. : Same as Q.8.(d) 2003
c) Degree of polymerization of a sample of poly methyl methacrylate ( PMMA ) is found to be 1000. What is the number average and the weight average molar masses of PMMA ? _
Ans. : PMMA is poly methyl methacrylate. It is a polymer of Methyl methacrylate.
O .
Ii -
CH2=C- C -och3
CH,
Miilt-cular weight of methylmethacrylate (M0) is 100. Degree of poly merisation - 1000 Number Average molecular weight Mn = - = qqq " 50050 (Ans.)
n-l 1000 - 1 999 .
where P " n + j " 1000 + 1 1001 0,998
1 + pn Weight average molecular weight Mw = Mo
1-Pn
1 + 0.998
= 100 x
1 - 0.998 100*,. I'm
. v 002 -.002
d) What is the importance of functional group region in IR spectroscopy ? What are the different absorption peaks possible for methanol and ethanol ? 1+3
Ans.Importance of Functional Group Region In Ifspectoscopy.
(a) To detect the functional group in the organic molecule.
, (b) "to detect the bonds present in the molecule. From the above we can determine the structure of a organic compound. ,
IR absorption peaks of ethanol (CHjCH2OH) : In ethanol three characteristic peaks are obtained.
(i) Due to O-H stretching One peak appearing at 3630 cm-1
(ii) Due to C-H stretching One peak appearing at 3000 cm-'1 %
(iii)Due to C-C Stretching one peafcappearing at <1000 cm-1 '
IR absorption peaks of methanol (CH3QH) :
11. a) Write notes on the following :
Joule-Thomson expansion and inversion temperature.
Ans : (a) Joule Thomson Expansion : When a gas is allowed to expand from one higher pressure to lower through the porous plug, which acts us a throttle in thermaly insulated apparatus so that the process is adiabatic. A lower temperature can be observed at lower pressure region and this cooling observed is found to be directly proportional to pressure difference. The above adibatic expansion of the gas often referred as Joule-thomson expansion and the temperature change upon expansion is known as Joule Thomson effect.
In case of Joule - Thomson expansion, dH = 0 '
We know, jp= Cp (Specificn heat at constant pressure) dh = 0
1
CP
H - Joule Thomson coefficient
when n = 0, T-b-0, r T> Rb 'T, = Inversion temp.
H and b are Vander Waals constant
For every gas there is a temperature when these is neither cooling nor heating observed and it is known as Inversion temperature T( ?
- 2a ' '
Thus inversion temperature of a gas, T; =
The co-efficient of J-T expension is important in the liquidation of gases because it predicts whether a gas cools or heats or expand. The co-efficient is a decreasing function of temperature ami it passes through & so at inversion temperature T,. In order to liquify a gas by J-T expansion, the gas must be cooled below the J-T inversion temperature.
Ans : (b) Chemical Potential (|A>- : The chemical potential of a given substance is the change in free energy (G) of the system when I mole of that particular substance, at constant temperture and pressure is added to such a large quantity of the system so that there is no appreciable-change in the overall composition of the system. We know G = f(P, T,n n2, n3........rtj)
We can write,
i N
dG 5nj
T. p.nj - Hi .......i) [ - Chemical potential of i th component]
The eqn (i) may written as : :
= dT+jJ dp + M,dn,+|t2dn2 + .........+ MidnJ
where j-tj, fi2......*.......are the respective chemical potential of the components I,
2, ... ........j -
If T and P remain constant, then
(dG)T P = |a,dn,+}*2dn2+.-.----+Mjdnj (iii)
If a system has definite composition having nl5 n2 ................ n, moles of the constituents
1,2,...............j respectively, then integration of (iii) gives ; -
.(G>r,p,nj +n22+............. njjij....................(iii)
So we can also say chemical potential may also be defined as the contribution per mole of each particular constituent of the mixture , to the total free energy of the system under condition of constant temperature, pressure and definite composition.
Significance of chemical potential in explaining equilibrium of reacting system.
Chemical potential is an intensive property and it may be regarded as the force which
drives the chemical, system to equilibrium. At equilibrium the chemical potential of the substance in the reacting system must have the same value through the system.
In other words, the matter flows spontaneously from a region of high chemical potential to low chemical potential. Chemical potential may also be regarded as the escaping tendency of that system, Greater the Chemical potential of a system greater will be its escaping tendency.
Condition for the equilibrium is : .dn, = 0 '
Ans. (c) Hydrogen bond : Same as Q.5 2004 v
Effccts of Hydrogen bond on properties of Compound :
(i) Boiling points of liquid compound in creases because of bydrogen bonding leads to increase in intermolecular attractions which leads to increase in heat of vapourization. eg. Boiling pt H20 (373K)> CH3OH (336K) > CH3OCH3 <268 K)
(ii) Solubility of organic compounds in water is.attributed to hydrogen bond formation eg. CH3OCH3 is completely miscible with water whereas CH3SCH3 is partially miscible.
(iii) Viscosity of liquid compound increases due to intermolecular hydrogen bonding.
(iv) Unique properties of water due to hydrogen bonding.
(d)Non-stoichiometric defects.
Ans. (d) Same as Q. 7. (a) '2003
Ans. (e) Carburetted wate gas : We know cracking of crude oil generates some hydrocarbons and if these gaseous hydrocarbons are mixed with water gas [CO(g) + H2(g)], calorific value of water gas increases. Hence Carburetted wate gas is a mixture of water gas and some gaseous hydrocorbons. This carburelted water gas contains about 35% H2, 25% CO, 25% N2 + C02, Its Calorific vlaue is about 45000 k Cal Am3 and it is used for heating and illuminating purposes. .
Semi water Gas : Same as Q. 11.2006 Q. II. F. Perfect and Inperfect Complex 5
Ans. : Question is Out of Syllabus.
|
Attachment: |
| Earning: Approval pending. |
