Vinoba Bhave University 2008 B.Sc Chemistry honours - Question Paper
Part-2
2008
VHB Ch (3) C
|
Choose the correct answer of the following questions: (a) Molar conductance is equal to : (i) Specific conductance X volume (ii) Specific conductance X pressure (iii) Equivalent conductance X temperature (iv) None of these (b) When pressure is increased for the equilibrium reaction N2(g) + 3H2(g) 2NH3, yield of NH3: (i) Increases (ii) Decreases (iii) Remains constant (iv) None of the above (c) Heat of neutralisation of strong acid and strong base is: (i) 9.0 K.cals (ii) 13.6 K.cals (iii) 12.6 K.cals (iv) 5.7 K.cals (d) If the length and cross-sectional area of a conductor are T cm and a cm2, then the cell constant is: (i) Ixa (iv) None of these 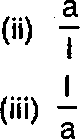 |
(e) Strong electrolyte is: (i) KOI (ii) CH3COOH (iii) NH4OH (iv) H2C03 2. Derive: (a) Cp Cv R (b) PV* = Constant 3. Define second law of thermodynamics. Discuss Carnot cycle and calculate its efficiency. 4. Define Phase, Component and Degree of freedom. Discuss water system. 5. Define and discuss consolute temperature. Explain Lower and Upper consolute temperature giving suitable examples with graph of all the three systems. 6. Define Equivalent conductance, Molar conductance and Specific conductance. How will you measure conductance of a electrolytic solution ? 7. (a) What is Kohlraushlaw ? Discuss with suitable examples. (b) Calculate degree of dissociation of acetic acid. Given : equivalent conductance at a given concentration Ac = 16.3 mhos cm2eq~1 and equivalent conductance at infinite dilution Xa = 390.7 mhos cm2 equiv _1. |
i) Calculate the free energy change accompanying the compression of 1 mole of C02 at 57C from 5 atm to 50 atm. Assume that C02 behaves like an ideal gas. 5) The solubility of AgCi is 1.435 x 10r-3 gm per litre at ordinary temperature. Calculate the solubility product of AgCI (Mol wt. of AgCI = 143.5) 9. Write notes on any three of the following : (a) Entropy (b) Gibbs-Helmholtz Equation (c) Desilverisation (d) Azeotropic mixture |
|
Attachment: |
| Earning: Approval pending. |
