University of Mumbai 2006 B.Tech Applied Science - II - Question Paper
Applied Science II May 2006 (Revised Course)
Section I
N.B. (1) Question No. 1 is compulsory. Attempt any two questions from Question Nos. 2 to 5.
(2) Attempt both the sections in separate answer books.
(3) Assume suitable data and symbols if required.
(4) Figures to the right indicate full marks.
1. Answer any four questions from following six questions: (20)
(a) Explain why in Newton's ring experiment the rings are unequally spaced? What will be the effect on the diameter of any ring if air gap between the lens and the plate is replaced by liquid?
(b) Explain the following :
i. Why is the cladding rarer than core in fiber optic cable?
ii. What is the measure advantage of graded index fiber over step index fiber?
iii. When is the use of laser preferred than LED in fiber optic communication?
(c) Write the difference between fission and fusion. What is stellar energy? Why it can't be produced on the earth?
(d) How Bragg's law can be used for analysis of crystal structures?
(e) Compare between ordinary source of light and a Laser. What is the Importance of metastable state and pumping in the production of Laser?
(f) A parallel beam of light ( X = 5870 A0) is incident on a thin glass plate ( = 1.5), such that the angle of refraction into the plate is 60. Calculate smallest thickness of the glass plate which will appear dark by reflection ?
2. (a) Explain the significance of Mosley's law. When an electron from the L-shell is transferred to K-shell, X-rays emitted have a wavelength of 0.788 A. What is the atomic number of this atom? Screening constant b = 1, Rydbergs constant R = 1.097 x 107/m. (8)
(b) What is diffraction grating ? What is grating element. A diffraction grating used at
Normal incidence gives a line (5400 A) in a certain order superimposed on the violet line
(4050 A0) of the next order. If the angle of the diffraction is 30, how many lines/cm are there in the grating ? (7)
3. (a) Explain with neat figure the construction and working of a Ruby laser. (8)
(b) In an uncontrolled fission reaction 1 milligram of U235fissions out in 1. microsecond. Calculate the power of explosion. Given that the energy produced per U235fission is 200
MeV. (7)
4. (a) Explain with figure the construction and working of G. M. counter used for counting nuclear particles. (8)
(b) A soap film ( = 1.33) on a wire loop held in air appears black at its thinnest portion when viewed by reflected light. On the other hand a thin oil film ( = 1.20) floating on water
( = 1.33) appears bright in its thinnest portion, when similarly viewed from the air above. Explain these phenomena.(7)
5. (a) The spacing between the principle planes in a crystal of NaCI is 2.82 A. It is found that first order Bragg's reflection occurs at 10. (8)
(I) What is the wavelength of X ray ?
(II) At what angle the second order reflection occurs ?
(Ill) What is the highest order of reflection seen ?
(c) Write the difference between Holography and Photography ? Explain the process involved to construct the hologram with proper figure. .(7)
Section II
N.B. (1) Question No. 6 is compulsory. Attempt any two questions from Question Nos.
7 to 9. (2) Atomic weights : C = 12, H = 1, S = 32, O = 16, CI = 35.5. N = 14.
6. Attempt any five from the following : .(20)
(a) What is cracking ? Explain the advantages of catalytic cracking over thermal cracking.
(b) Explain scope and importance of biotechnology in medicine and agriculture.
(c) . Explain how sulphur can be estimated in coal.
(d) Write a note on Pitting Corrosion.
(e) Explain how you would control corrosion by improvement of design.
(f) A sample of coal has following composition by mass C = 70%, O = 8%, H = 10% N = 3%, S = 2%, Ash = 7%. Calculate H.C.V. and L.C.V. using Dulong formula.
(g) Give the composition, properties and uses of Duralumin.
7. (a) How do the following factors influence the corrosion rate .(8)
(i) Position of metal in glavanic series (iii) Temperature
(ii) Relative areas of anode and cathode (iv) pH of the medium.
(b) What is Petroleum ? How is crude petroleum refined ? Name various products obtained from it ? .(7)
8. (a) i) What are plain carbon steels ? How are plain carbon steels classified on the basis of their carbon content ? .(2)
(ii) Write composition, properties and uses of (a) German Silver (b) Nichrome. .(6)
(c) Explain the manufacture of biogas from waste material. Give composition, properties and uses of biogas. (7)
9. (a) Mention the various methods of metallic coating and explain any two of them. .(8)
(b) (i) Calculate the weight of air needed for complete combustion of 1 gm of coal containing .(4)
C = 72% H = 10% 0 = 9% N = 3% and remaining being ash.
(ii) Explain knocking and role of antiknocking agents in gasoline. .(3)
Applied Science II November 2005 (Revised Course)
Section I - (Marks: 50)
N.B. (1) Question No. 1 is compulsory.
(2) Answer any four questions from remaining six questions.
(3) Assume suitable data whenever required.
(4) Figures to the right indicate full marks.
(5) Illustrate your answer with sketches wherever necessary.
1. (a) Calculate the least width that grating must have to resolve the components of Sodium D
lines in the second order, the grating having 1000 lines/cm. D1= 5896 A and D2= 5890 A. (5)
(b) In Newton's rings experiment, the diameter of a certain bright ring is 0.65 cm and that of tenth ring beyond it is 0.95 cm. If wavelength of the source used is 6000 A,
calculate the radius of curvature of a convex lens surface in contact with the glass plate. (5)
2 (a) Describe the process of obtaining a three dimensional image by the holography method. .(5)
(b) When a slow neutron is captured by U235nucleus, fission results in the release of 200 MeV energy. If the output power of a nuclear reactor is 1.6 mW, calculate the number of Nuclei undergoing fission per sec. .(5)
3. (a) Discuss the phenomenon of Fraunhofer diffraction at single slit and obtain condition for first minimum in the pattern. .(5)
(b) In thermonuclear reaction 1.09 x 10 -3kg of hydrogen is converted in to 0-983 x 10 -3kg of helium. .(5)
(i) Calculate energy released in joules.
(ii) If the efficiency of the generator is 5%, calculate the electrical energy output in kWH.
4. (a) Derive the expression for Numerical Aperature for fibre optics cable.
What do you understand by acceptance angle and what is it's significance ? .(5)
(b) If the p.d. applied across an X-ray tube is 25 kV and current through is 10 mA, calculate no. of electrons striking the target per second and minimum wavelength limit for the X-ray generated. .(5)
5. Write short note on (any two): 10
(a) G.M.Tube (c) Nuclear Chain Reaction.
() Mass defect and binding energy
6. (a) A wedge-shaped airjilm, having an angle of 45 seconds, is illuminated by monochromatic light and fringes are observed vertically through a microscope. The distance measured between the consecutive film is 0.12 cm. Calculate the wavelength of light used. 5
(b) The atomic mass of 8016is 16 a.m.u. Find out binding energy per nucleon. 5
(Given: mass of Electron: 0-00055 a.m.u.) mass of Proton : 1 -007593 a.m.u.) mass of Neutron : 1 -008982 a.m.u.)
7. (a) What is laser action ? Explain the following terms with their specific importance in laser action: 5
(i) Optical pumping (Hi) Metastable state.
(ii) Population inversion
(b) Explain what is step, graded index, mono-mode, multimode fibre. 5
Section II - (Marks: 50)
N.B. (1) Question No. 1 is compulsory.
(2) From remaining five attempt any four questions.j
(3) All questions carry equal marks.
(4) Figures to the right indicate full marks.
1. Attempt any five from the following: 10
(a) Name few characteristics of a good fuel.
(b) Give the composition and calorific value of Biogas.
(c) Give composition and uses of Duralumin.
(d) What are plane carbon steels ? How are they classified on the basis of carbon contents ?
(e) Distinguish between anodic and cathodic metallic coating.
(f) Give the classification of crude oil.
2. (a) What is corrosion ? Explain the corrosion due to combination of different metal electrodes.
4
(b) Write the composition, properties and uses of any two of the following : 6
(i) Germal Silver (ii) Type Metal (iii) Commercial Brass.
3. (a) Give a brief account of various types of coal. Write their composition and calorific value. 6
(b) Calculate the gross and net calorific value of coal having following composition: 4
C = 80%, H = 7%, O = 3%, S = 3-5%, N = 2-1 % and ash = 4-4%.
4. (a) What is cracking ? Distinguish between thermal and catalytic cracking. 4
(b) Explain the preparation of ethyl alcohol from sugarcane. 6
5. (a) Write short note on any two of the following : 6
Cathodic protection by impressed current
Water line corrosion
Special Steels.
(b). A sample of coal containing 5% H2when allowed to undergo combustion in bomb calorimeter, the following data were obtained:- 4
(i) Wt. of coal burnt = 0.95 gm
(ii) Wt. of water taken = 700 gm
Water equivalent of bomb and calorimeter=2000gm
(iii) Rise in temperature = 2.48C
(iv) Cooling correction = 0.02C
(v) Fuse wire correction = 10.00 Cal
(vi) Acid Correction = 60.00 Cal
Calculate gross and net calorific value of coal.
6. (a) What are the main constituents of paint ? Write their functions. 4
(b) A gas has the following composition by volume H2= 20%, CH4= 6%, CO = 22%, C02= 4%, 02= 4% and N2= 44%. Find the volume of air actually supplied perm3of this gas.
Applied Science II June 2005 (Revised Course)
Section I
N.B. (1) Question No. 1 is compulsory.
(2) Answer any four questions from remaining six questions.
(3) Assume suitable data whenever required.
(4) Figures to the right indicate full marks.
(5) Illustrate your answer with sketches wherever necessary.
1. (a) Show, with clear examples, that separation between two consecutive'similar rings, in
Newton's Rings Experiment, goes on reducing as the serial number of ring increases.
5
(b) In Newton's Rings experiment, the nthdark ring due to light.of wavelength ![]()
coincides with (n+2)thdark ring due to light of wavelength ![]() Show that radius on nth
Show that radius on nth
dark
ring is given by 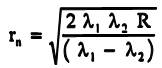
2. (a) Explain diffraction due to a single slit. Arrive at conditions for
maxima and minima.Take as ![]() wavelength of incident
monochromatic light. 5
b) Calculate the highest order of spectrum that can be obtained by a monochromatic light
of wavelength 6000
A0by a grating of 6000 lines
per centimeter. 5
wavelength of incident
monochromatic light. 5
b) Calculate the highest order of spectrum that can be obtained by a monochromatic light
of wavelength 6000
A0by a grating of 6000 lines
per centimeter. 5
3. (a) Explain with neat sketch optical fiber communication link. 5
(b) Internal critical angle in core at core-cladding interface of a step index optical fiber is 80.6. Calculate maximum acceptance angle if refractive index of cladding is 1.48. 5
4. (a) Explain construction and working of HeNe laser with neat energy level diagram. 5
(b) Find how many photons are required to produce laser beam of 3 mW power,
if wavelength of the same is 6943 A0. 5
5. (a) Differentiate between continuous X-rays and characteristic X-rays. 5
(b) Explain Moseley's law and its significance. 5
6. (a) Describe various parts of a nuclear reactor and their functions. 5
b) Explain the term "binding energy per nucleon." Find binding energy per nucleon of 3Li7, if mass of proton = 1.0081437 amu, mass of neutron = 1.0089830 only and
mass of 3Li7= 7.016005 amu. 5
7. Write notes on any two : 10
(a) Antireflection films (2) Coolidge Tube (3) Holography.
Section ll
N.B. (1) Question No. 1 is compulsory.
(2) Answer any four questions fronvremaining six questions.
(3) Figures to right indicate full marks.
1. (a) Give the applications (any 4) of Biotechnology in agriculture. 10
(b) What is the role of ash in coal ?
(c)- Give the characteristics of good quality paint ? (any 4)
(d) What is metal cladding ?
(e) Give composition and properties of Duralumin.
(f) Select the compound which has the highest octane No. and highest cetane No. out of n-heptane, n-hexadecgne, n-octane, iso-octane.
2. (a) What is power alcohol ? How is it obtained from molasses ? 5
(b) 2.9 gm of coal was heated in electric oven at 110CC. The weight of sample gets reduced to
2.75 gm. Further heating at 925C for 7 min. in muffle furnace, with lid, reduces the wt. of sample to 2.45 gm. This sample on heating at 750CC, for 1/2 hr., gives residue of 0.13 gm. Calculate % C.
3. (a) Why galvanisation of articles is preferred to tinning ? 4
(b) A gas has following composition by volume : 6
CH4= 40%. C2H6= 20%, C3H8= 10%, H2= 10%, CO = 10%, N2= 10%. Calculate weight of air required per m3of gas. (Mol wt q air = 28.94)
4. (a) What is the effect of following elements on alloying steel-: (any two) 4
(i) Cobalt (ii) Tungsten (iii) Nickel
(b) Explain differential aeration corrosion with example. - 6
5. (a) How are the following factors responsible for rate of corrosion : (any three) 6
(i) Relative areas of Anode and Cathode (ii) PH (iii) Overvoltage
(iv) Nature of surface film.
(b) Give well labelled diagram of Bio-gas with calorific value and composition. 4
6. (a) What .is cracking and Explain Moving Bed Catalytic Cracking. 6
(b) Give an. account of alloys of lead and tin. 4
7. (a) What do you mean by impressed current cathodic protection ? 4
(b) Explain octane rating and role of antiknock agents in Gasoline. 6
| Earning: Approval pending. |
