University of Hyderabad (UoH) 2008 Entrance Exams Other Entrance Exams Integrated m.sc entgrance - Question Paper
Integrated M.Sc. Entrance Examination -2008
MM Marks : 100 Time : 2 hrs
Hall Ticket No.
INSTRUCTIONS
I. Please enter your Hall Ticket Number on this page and on the OMR sheet without fail.
II. Read the following instructions carefully.
1. Questions 1-25 are in Chemistry, 26-50 i]ft Physics, 51-75 in Mathematics and 76-100 are in Biology.
2. Answer as many questions as you can. Each question carries 1 mark. Each wrong answer will be awarded -0.33 mark. The total marks for the written examination will be scaled to 75.
3. Answers are to be marked on the OMR sheet following the instructions given there.
4. Hand over both the question paper and the OMR sheet at the end of examination.
5. Non-programmable calculators are allowed. Log tables and calculators are not allowed.
6. Rough work can be done anywhere on the question paper butvnot on the OMR sheet.
7. This book contains 22 pages including this page and pages for the rough work. Please check that your question paper has all the pages.
[A] Na2[Fe(N0)(CN)43.2H20 [B] Na2[Fe(N0)(CN)5].2H20
[C] Na3[Fe(N0)(CN)4].2H20 [D] Na3[Fe(N0)(CN)5],2H20
The element with the electronic configuration ls22s22p63s23p63d74s2 is [A] Manganese [B] Iron
[C] Cobalt [D] Rhodium
Electrolysis of brine produces
[A] Chlorine and sodium [B] Chlorine and hydrogen
[C] Sodium hydroxide and chlorine [D] Hydrogen* chlorine and sodium
hydroxide
Volume of 0.75M NaOH solution needed to neutralize 75 ml of 0.25M H2S04 is [A] 25 ml [B] 50 ml [C] 75 ml [D] 100 ml
H2S is a weak acid that ionizes as H2S -+ 2H+ + S2'. Addition of a few drops of HC1 will
[A] Decrease the concentration of S2\
[B] Increase the concentration of S2\
[C] Have no effect on the concentration of S2\
[D] Raise the pH value alone.
The noble gas element having ionization potential close to that of oxygen molecule is
[A] Argon [B] Krypton [C] Xenon [D] Radon
The oxygen content in metal oxide is 47%. The gram-equivalent weight of the metal would be
[A] 3 [B] 6 [C] 9 [D] 18
The values of x andy that balance the following reaction are MnO4- + xlV +yFe2* - Mn2* +>>Fe3+ + (x/2) H20
[A] 4 and 6 respectively [B] 6 and 3 respectively
[C] 4 and 8 respectively [D] 8 and 5 respectively
The maximum number of electrons that can be accommodated in a shell with principal quantum number n is
[A] 2n [B] 2n2 [C] 2n3 [D]2n4
*
A reaction vessel contains 14.0 gm of N2 and 3.0 gm of H2 at 10 atm. pressure. The reaction is carried out until all the nitrogen is converted to ammonia, and the system is brought back to the original temperature. The final pressure in the vessel would be
[A] 5 atm. [B] 7 atm. [C] 9 atm, [D] 11 atm.
The first ionization potential of three successive elements in the periodic table are 1086,1402, and 1314 kJ/mole respectively. These elements would be
[A] Be, B, C [B] B, C, N [C] C, N, O [D] N, O, F
5 ml of 0.4M HC1 and 5 ml of 0.2M NaOH solutions are mixed together. The concentration of H+ ion in the resulting solution is
[A] 0.1M [B] 0.2M [C] 0.3M [D] 0.4M
14. The solution with the highest saturated water vapor pressure over it is [A] 0.5M glucose solution [Bj 0.2M MgSO< solution
[C] 0.3M Na2S04 solution [D] 0.2M K3PO4 solution
15. An example of a molecule that contains both types of chemical bonds (ionic and covalent) is
[A] HC1 [B] NaCl [C] CH3C1 [D] NH4C1
16. The molecule in which oxygen carries +1 oxidation state is
[A] F2O2 [B] F20 [C] H202 [D] H2O
17. When 1.2 gm of a hydrocarbon was burnt completely, 3.3 gm of CO2 and 2.7 gm of water were obtained. The empirical formula of the hydrocarbon is
[A] CH4 [B] CH, [C] CH2 [D] CH
18. The atom with the largest atomic radius among the following is '
[A] F [B] Ne [C] Na [D] Mg
19. The first organic compound artificially prepared from inorganic starting material is
[A] acetic acid [B] urea
[C] formic acid [D] methane
20 Beilsteins test is used for the detection of
[A] nitrogen [B] sulfur [C] halogens [D] phosphorus
[A] CH4 [B] H20 [C] NH3 [D] bf3
The alcohol that gives the most stable carbocation intermediate is
[B] CH3CH2CH2CH2OH
[A] C(CH3)3OH
[C] CH3CH2CHOHCH3
[D] (CH3)2CHCH2OH
The reagent that is useful for separating benzoic acid from a mixture of benzoic acid and phenol is
[B] dilute HN03
[A] dilute HC1
[D] NaHC03 solution
[C] NaOH solution
Stability of the alkyl carbocations is determined by
[A] Inductive effect
[B] Hyperconjugation
[C] Both the inductive effect and hyperconjugation
[D] Electomeric effect.
The compound with zero dipole moment among the following is
[A] 1,1 - dichloroethance [B] 1,1- dichloroethene
[C] cis -1,2 - dichloroethene [D] trans - 1,2 - dichloroethene
[A] 5.56 cm [] 4.43 cm
[C] 4.13 cm
[D] 3.74 cm
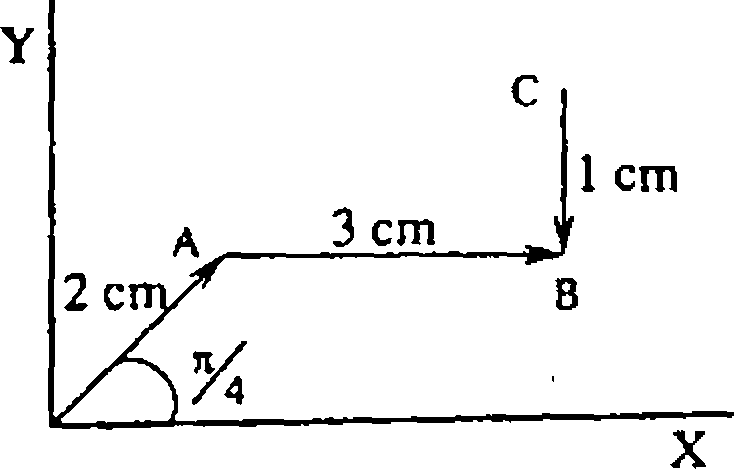
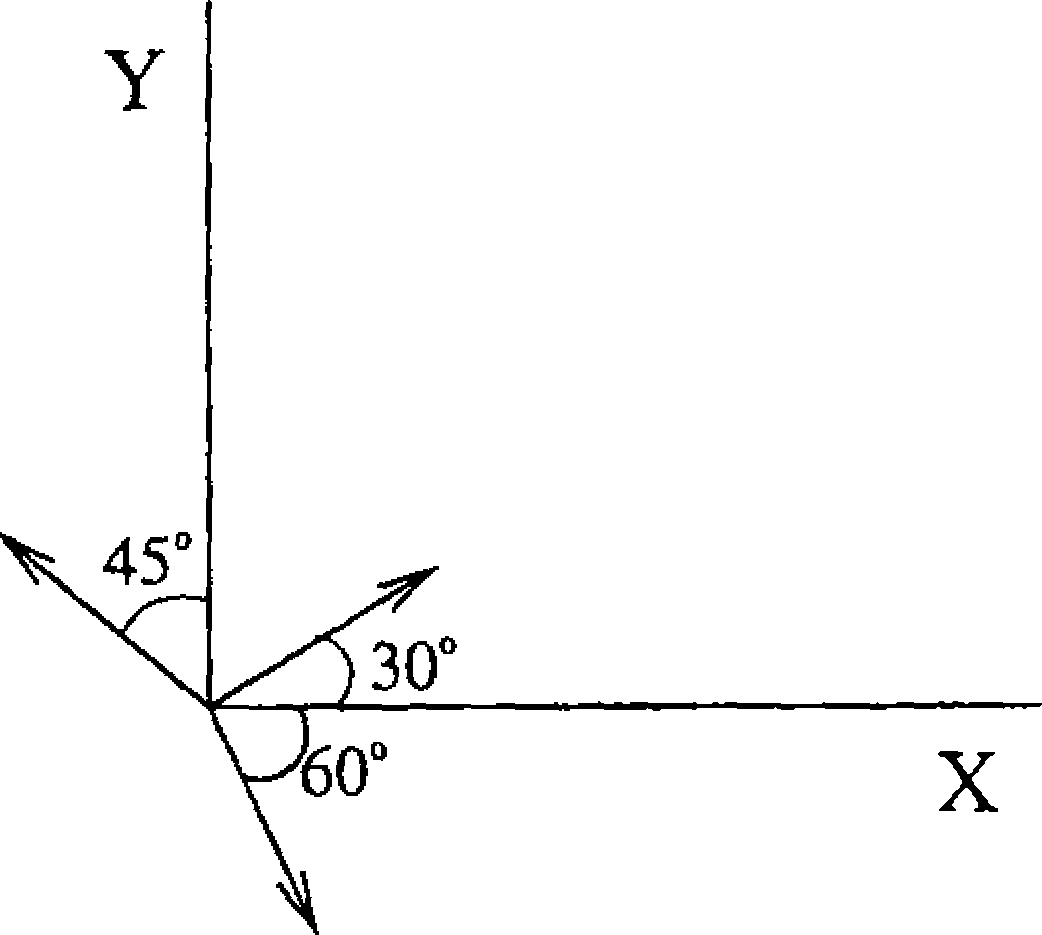
27. The magnitudes of vectors OA, OB, and 0Cy are equal. The vector OA + OB -OCy makes an angle 9 with X - axis. The value of 6 is,
 |
+ x/3 + n/2 y/3 + y/2 \/3 \/2 -j- y/3 4* y/2 -y/S + y/2 |
- V3- y/2
+ Vd + V2
+ y/3 y/2
28. A man can swim at a speed of 3 km h"1 in still water. He wants to cross 500 m wide river flowing at 2 km h"1. He keeps himself at an angle of 120 with the river flow. The time taken by him to cross river is
[D] 9.5 min
[C] 10.3 min
[] 11.5 min
[A] 20.7 min
29. Three particles weighing 1 kg, 2 kg, and 3 kg are placed at the corners of an equilateral triangle of side 1 m as shown in the figure. The position of the centre
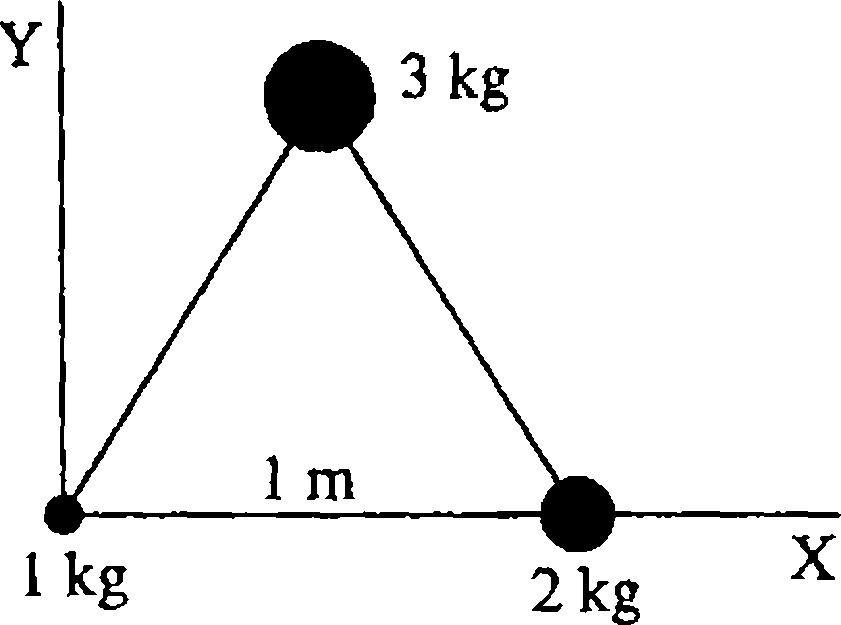
|
denoted by (xq , | |
|
7 |
_ v'a |
|
~ 12 ~ |
4 |
|
3 |
v'S |
|
= 4 * - |
24 |
|
y/S |
1 |
|
= r> yQ |
6 |
|
i |
1 |
|
- 12> Vo - |
12 |
30. A body of mass mi moves toward another body of mass m2 which is at rest. After the collision, it is never possible that
[A] both the bodies move after collision in same direction
[] both the bodies move in opposite direction after collision
[C] the moving body comes to rest and the stationary body starts moving
[22] the stationary body remains stationary, the moving body changes its velocity
31. The adjoining figure shows the graph of the position of a particle moving along x-axis as function of time. The average velocity vf during 0 to 10 sec is
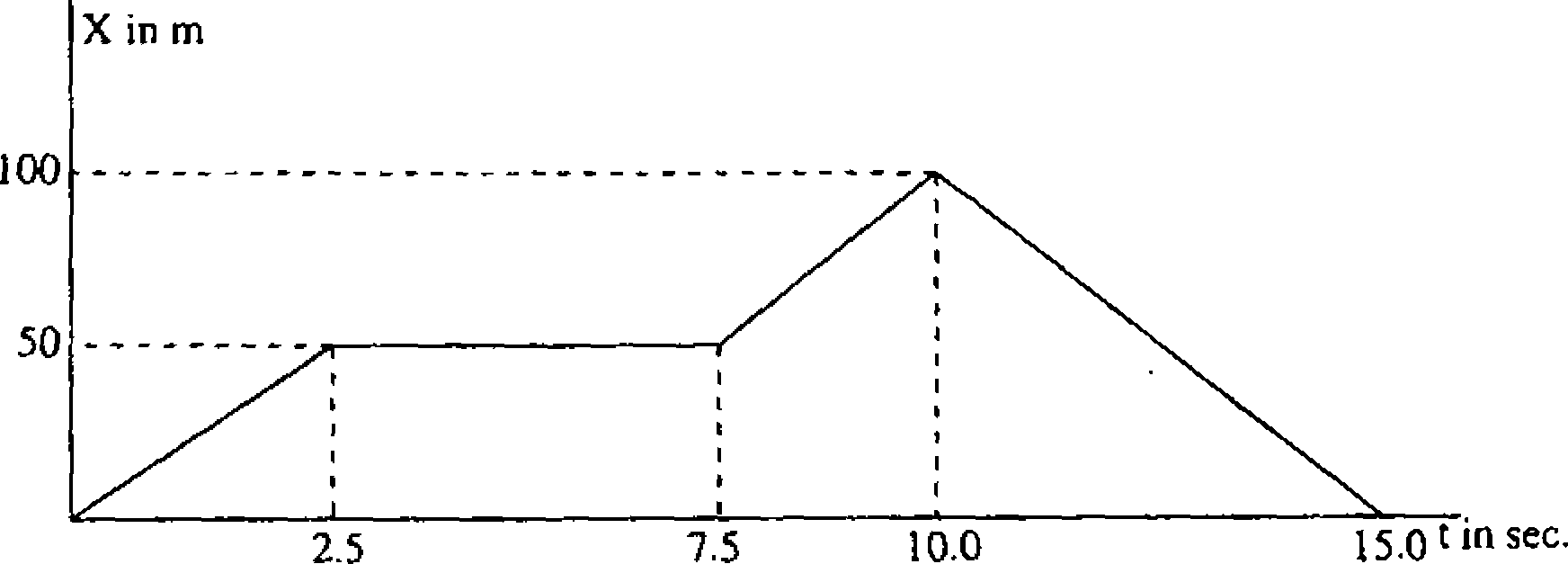
|
-1 |
32. A sonar on the belly of a helicopter at a height of 500 m above sea level, sends a sound signal perpendicularly down to find the depth of ocean below. The reflected sound signal from bottom of ocean is received 5 s after the transmission. The speed of sound in water is 1.5 times that in air. The depth D of the ocean is,
|
[A] D < 0.3 km |
[C] 0.6 < D < 1.9 km [] 0.3 < D < 0.6 km [D] D > 1 km |
33. A lab purchases four different tuning forks. To find the unknown frequency of one of the forks, a student strikes it and attaches one prong to a wire with a linear mass density 3 kg m1, the other end of which is attached to a mass 10 kg going over a pulley. The length of the wire from the prong to the pulley is 5 cm. He observes the wire not moving at 3 points (in addition to fixed ends at fork and at the pulley). To which of the following categories the tuning fork belongs ?
[A] forks of frequencies 205 215 Hz
[B] forks of frequencies 216 225 Hz
[C] forks of frequencies 226 235 Hz
[D] forks of frequencies 236 245 Hz
34. A point charge q is placed at (0,0). Another point charge 4q is placed at (d, 0). A test charge is placed at (x, 0) so that it is in equilibrium. The value of x is,
r , (I
[C] +d
[.D] -d
35. In the shown electric field, a positive charge is moved from Point A to point B. Its potential energy -
B
[A] increases
[B] decreases a
[C] remains constant
[D\ changes depending on the path taken from A to B
36. A dipole is placed inside a sphere of radius R. The clectric field flux coming out of the sphere is
[B] 2/c0
(n) 1 1 1
[A] 1 /t0 [C] zero
37. The equivalent resistance between the points A and B is
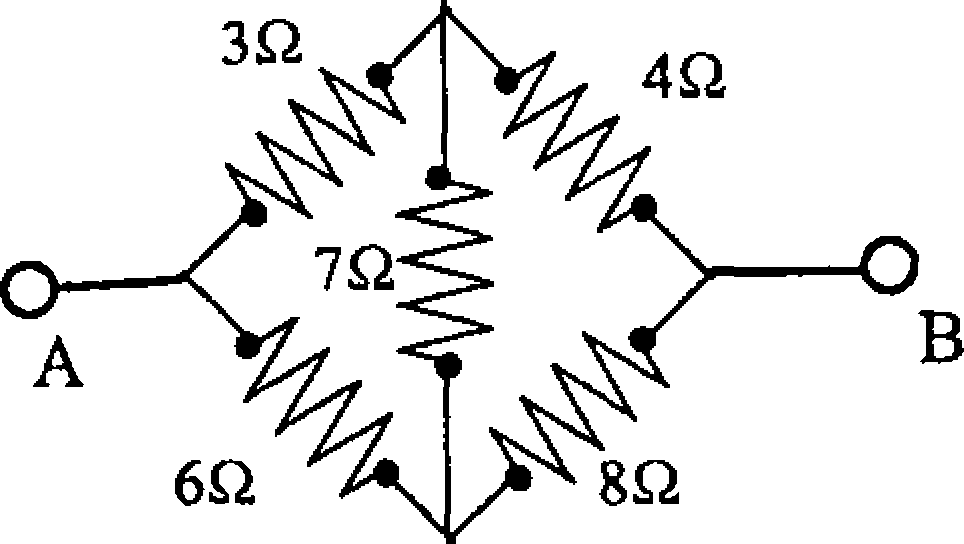
|
26 n | |
|
\B] |
7.3 n |
|
[C\ |
4.6 n |
|
2.8 n |
38. A small circular loop of radius r is placed inside a circular loop of radius R (R > r). The loops are coplanar and their centres coincide. The mutual inductance of the system is proportional to
[Cl
[D]
R2
R?
r2
~R
39. Two alternating currents are given by I\ ~ /0 sin ut and = h cos(3cjt + 0). The ratio of their rms value is
W-o
40. An A.C. voltmeter reads 10 volt; then the peak value is
(y4] 14.1 Volt [] 10.07 Volt [C] 7.1 Volt [>] 5.0 Volt
41. Three circular concentric wires of radii r, 2r, and 3r are carrying current 3/, 2/, and / respectively in the same direction. The magnetic field at the center is
6r or ' ' r
42. Which of the following could not be used to indicate a change in temperature.
[A] change in colour of a metal rod
[?] change in length of a liquid column
[C] pressure of a gas at constant volume
[)] mass of one moi of gas at constant pressure
43. A mass m of helium gas is in a container of volume V. The gas is initially at pressure P and temperature T. Helium gas is added to the container until its mass is 3m. The temperature of the gas is now 2T. What is the pressure of the helium
?
gas [A] 6P
mg
\B)\P
44. For an isothermal process in an ideal gas, which of the following is always true
[A] no heat flows in or out of the gas
[B] pressure does not change
[C] volume does not change
[D] internal energy does not change
45. The snapshot of a wave (propagating in the positive x direction) taken at time t = 0 is shown in the figure. This wave can be represented by the expression
[A] A sin (ut + kx)
[J5] Acos(u/ fcc)
[C] As\n(kx wt)
[D] A cos (kx + wt)
13 ii0I
MtJ
[D] 0
[C]
[C] 2P
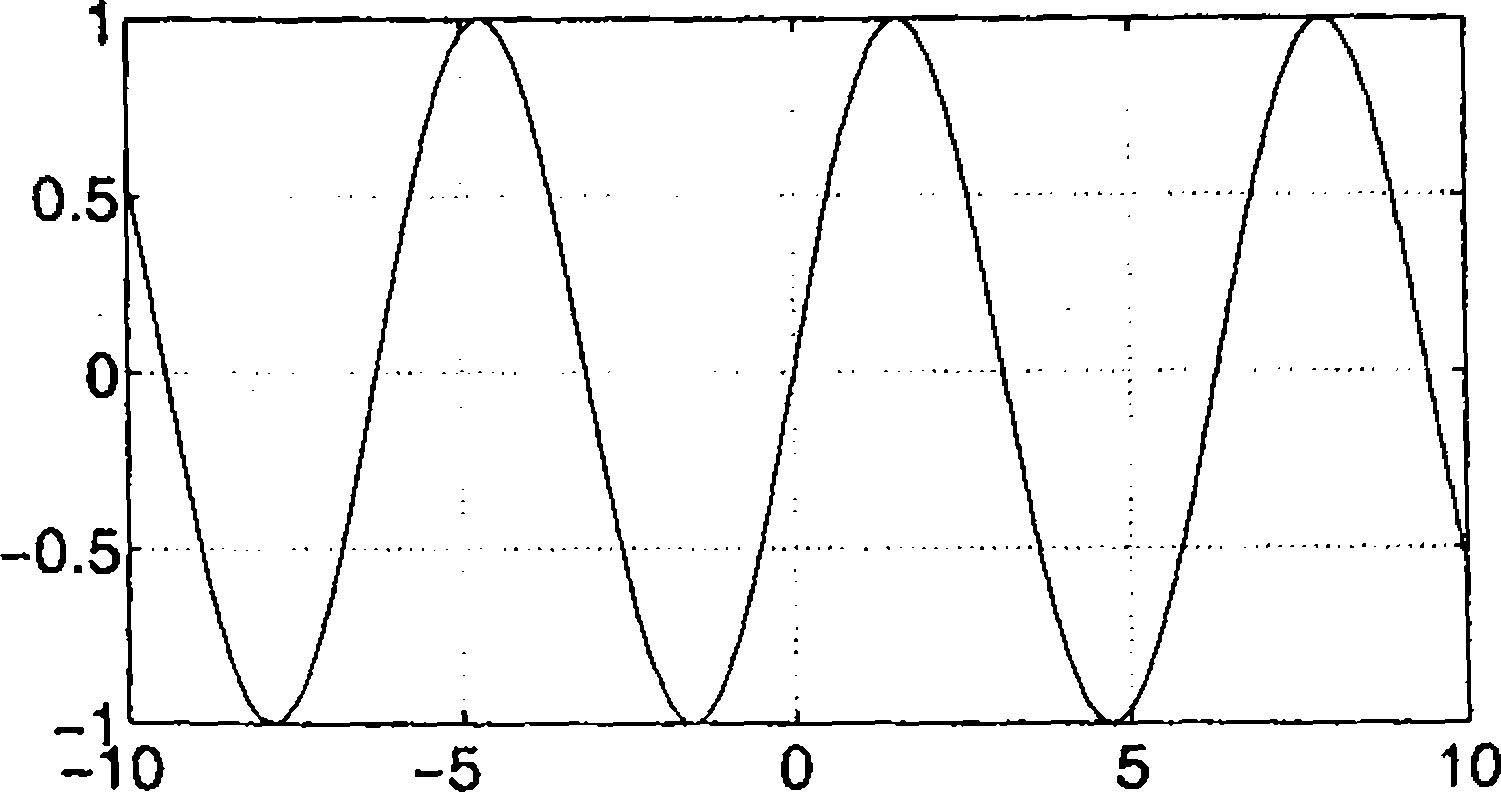
46. The plot of the displacement y A sin(27rft + 4>) in arbitrary units as a function of time (in seconds) is shown in the figure. This oscillation is characterized by
|
A = 1.5, |
/ mHz, |
0 = |
0 | |
|
[B] |
A = 1.5, |
f = 100 Hz, |
<p = |
7r/2 |
|
\<A |
A = 1.5, |
/ = mHz, |
0 = |
jr/2 |
|
[D] |
A= 1.5, |
** o o CM 1! |
0 = |
-ff/2 |
47. A ray of light is incident at an angle 0 on one of the two perpendicular mirrors as shown in the figure. If the incident and emergent rays are parallel, then
[A] 0 < 6 < tt/2
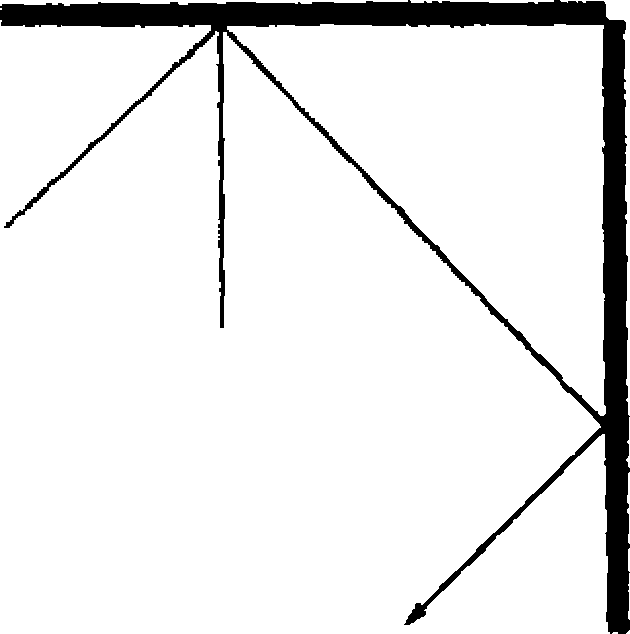
[B] 0 = 30
[C] 0 = 45
[D] 9 = 60
48. The superposition of two oscillations given by y J4(sin(wii) +sin(o>2i)) is periodic if only the frequencies satisfy the relation
[A] is a real number
[5] U1/0J2 must be an integer
[C] must be a rational number
[D] wx/cj2 must be an irrational number
* 49. Let a light beam be incident on a sphere with refractive index n = \/3 at an angle i from air and emerges parallel to the horizontal axis passing through the center of the sphere, see figure. Then
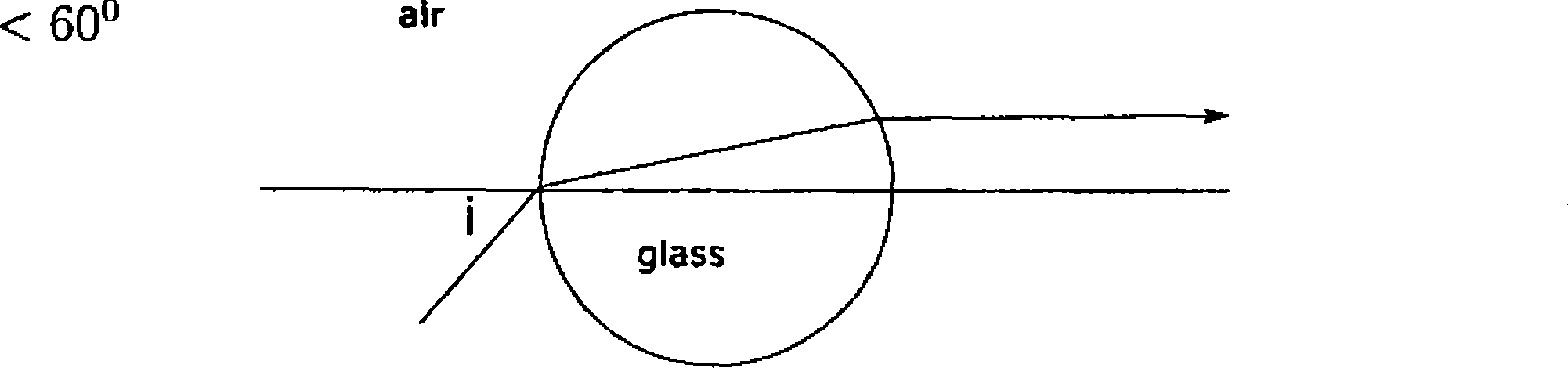
[C] i = 45
\D] i = 30
50. The intensity of each source Si and S2 arriving at point P is /o (see figure). Let the wavelength A=4 m. The total intensity at point P is given by
[A] 2/o
si
|
3 m | 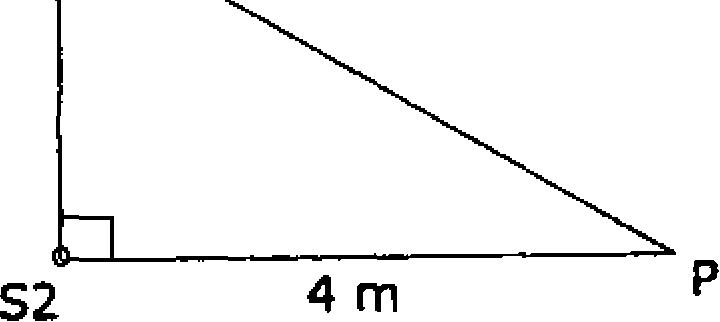 |
[C] 0
2 j.
51. The function f(x) = rj- is well defined on
[A] -00 < x < 0 [B] 0 < x < 00
[O] 0 < x < 1 [D] \/2 < x < 00
52. The value of
00
nkx> ' k2 + n2 k=1
equals
[A] 1 [B] 5 [C] f [D] 2
11
dU
53. If x ~ e-t, y = sin t and z = cos t and u = ln(x -f y + z)2 then =
dt
(cos t -f sin t e e)2 cost + sin + e_t
cos t + sin t + e_t (cost + sin e~f)2
I 4* 1 -f I 4- 4-
cos t sin t e t 2{cos 4- sin 4- e~l)
2(cos t sin t - e*) cost 4- sin t +
(5]
U>]
w
\o\
54. lim
n-oo 0.25n2 + n 4- 3
[C] -oo and A2 = B then a is
p] 0
[.B] oo
1 0 0 1
a 0 1 1
and B
[jD] none of these.
[C] 4
(B) -1
56. The value of ( i19
is given by
,-'25
ID] -4
[C] 2
[S] -2
(\/2+l)0 (v/2-l)0 ,
57. The only values of 6 in the range [0,21 for which cos---. cos---= 1
[i4] only 0
[D] 0 and 2tt
[C] 0 and
[] 0 and 7r
OO / n\ n
58. The sum of the series ( -
is
n=l
[D] I
IB] |
[Cj 1
59. The area of the region bounded by the curves y = (xl)2, y = 0, a; = 2, rr 4 is
11 f
P] f
[C] 9
60. Five red and three blue balls are to be arranged in a row; the number of arrangements possible so that there is a red ball at both ends is
61. There are seven fair coins. Each is tossed seven times. The probability of getting at least 25 heads altogether is
[C] To [D] \
62. The range of the function f(x) = + 3, x 0, is
[A) {1} IB] {2,4}
[O] [2,4] ID] (2,4)
63. The set {x <E: |x2 - 26| < 10} f){x : \x2 - 5| < 4}
[A] is a closed interval.
[B] is a nonempty open interval.
[C] is empty.
[D] contains exactly 2 points.
64. Suppose / and g are two differentiable functions such that ff(x) f(x) and g(x) = f'(x). If F{x) = f(x)2 + g(x)2 and F(0) - 7 then F(7) =
[A] 0 [S] 7e14 [C] 49 e~14 [DJ 14e14
65. If the cube roots of unity are 1, lo, oj2 then the roots of the equation (z + l)3 + 8 = 0 are
[A] 3, 1 4- 2w, 1 + 2w2 [?] 3o, 2 (dy 1 2to2 [C] 3, to2 + u>, oj + lo2 [?] 3 u>2, 1 + 2a;, 1 *+* 2 u2
66. If the roots of the equation X2 bX + c = 0 are two consecutive integers then b2 4c
[A] b + c [B]b-c
[C] 2 [D] 1
if n 1 is divisible by 3
if n 2 is divisible by 3
f(n) = <
n-f-I V 3
IS
|
[A] not defined for all n G N. [C] defined and onto N. |
[B] defined and one-to-one on N. [D\ defined on N and not onto. |
(a -f b)' a2 b2
c
(b + c)2 b2
68. The value of
or
(a + c)2
is
[A] 2abc(a -1- b + c)3
[5] (a + 6)2(6 H- c)2(c + a)2
[C] (a + 6)2(6 + c)2(c + a)2 - 3a262c2
[/}] (a + b)2(b + c)2(c + a)2 - 2a2&2 - 2b2c2 2c2a2
69. Let t > 2 be such that the area enclosed by the lines x = l,i/ = 0, x = 2 and the curve y = x2 is the same as the area enclosed by the lines x ~ 2, y = 0, x ~ t and the curve y = x2. Then t
[A] 3
[C] 15:
70. On the interval [-1,1] the function f(x) xz 3x has the maximum value M and the minimum value m. Then which of these numbers is closest to the length of the interval [m, M].
[A] 4.4 [B\ 4 [C\ 3.6 [D\ 3
71. The radius of the circle circumscribing the square whose vertices are (0,3), (1,0), (3,4) and (4,1) is
[B] between 1 and 2. [D] 4
[A] between 0 and 1. [C] between 2 and 3.
M-o|
72. If ro is a positive integer such that the coefficient of x4 in the expansion of (1+mrr)10 is divisible by 1000 then
[A] m is a multiple of 10. [B] m is a multiple of 5
[C] m is at least 10. [D] m is at most 1000.
73. The volume of the solid generated by revolving the region bounded by the curves y y 0 and x = 4 about the yaxis is
r 1.5
74. The value of / [x2] dx\ where [x] is the greatest integer function is
Jo
[A] 2+V2 [B] -2+22 [C] -2+x/2 [D] 2-\/2
75. An integer solution of (1 i)* = 2 2 is given by
[A] 8 [B] 1 [C] 2 [D] 6
BIOLOGY
76. A characteristic feature of gram-negative bacteria
[A] Thick peptodoglycan
[B] Ability to produce primarily exotoxins
[C] Highly susceptible to penicillin and sulfonamide
[D] Highly susceptible to streptomycin, chloremphenicol and tetracycline
77. Diameter of an eukaryotic cell is typically in the range of
[A] 0.2 - 2 pm [B] 1- 5 pm [C] 10 - 100 pm [D\ 100 - 250 pm
78. The first class of antibodies to appear after exposure to an antigen
[A] IgD [B] IgE [C] IgG [D] IgM
M - o
[A] 0. 5 nm [] 2. 0 nm
[C] 10 nm
\D] 20. 0 nm
80. Fibroin is a
[] Polydischaaride
[/}) enzyme
[A] Protein
[C] Long chain fatty acid
81. Although there are a limited number of amino acids, many different types of proteins exist because the
[A] size of a given amino acid can vary
[B] chemical composition of a given amino acid can vary
[C] sequence and number of amino acids is different [>] same amino acid can have many different properties
82. A recessive disorder is inherited
[A] 25%
[C] 75%
[B\ 50 %
[D] 100%
83. A healthy individual is a carrier of a lethal allele but is unaffected by it. What is the probable genotype of this individual?
[A] two dominant normal alleles
[] one recessive lethal allele and one dominant lethal allele
[C] one recessive lethal allele and one dominant normal allele
[D] one dominant lethal allele and one recessive normal allele
84. What portion of a plant the saffron herb represents
[A] Dried carpel [B] Dried stamen
[C] Dried roots [D\ Dried veins
85. Sickle Cell anemia is a disease
[A] X-linked recessive [] X-linked dominant
[C] Autosomal-dominant [D] Autosomal recessive
86. A leucocyte that is involved in anti allergic and healing of wounds
[A] Basophils [B] Acidophils
[C] Lymphocytes [D] Monocytes
[A] Glutamine [) Serine
[C] Aspargine [>] Proline
88. Lysosomes contain
[A] Ligases
pi Lyases
[C] Lipases
[D] Hydrolases
89. Which of the following phases represent the correct order in the prophase I of
karyokinesis-1 of the reductional division or heterotropical divison
[v4] Leptotene, Zygotene, Pachytene, Diplotene, Diakinesis
[5] Leptotene, Pachytene, Diplotene, Zygotene, Diakinesis
[C] Leptotene, Diplotene, Pachytene, Zygotene, Diakinesis
[.D] Leptotene, Pachytene, Zygotene, Diplotene, Diakinesis
90. Red Cedar, Jumperus virginiana, is
\A] An angiosperm, monocot [J9] An angiosperm, dicot
[C] Belongs to Sphenopsida of pertidophyta
[D] A gymnosperm
91. Promoter is a
[A] RNA coding sequence in DNA [] Protein coding RNA sequence
[C] Protein that regulates transcription
[D] Regulatory DNA sequence that plays a role in DNA to RNA synthesis
92. Most protein-coding RNAs in euaryotes
[A] are polycistronic [5] have 5 phosphate
[C] have 3 hydroxyl group [>] have 5 methylated guanylate
93. The principal use of Phase-contrast microscopy is
[A] to provide three dimensional image
\B] to facilitate detailed examination of the internal structures of living specimens
[C] to observe detailed examination of stained specimens
[D] uses a laser light to obtain two and three-dimensional images of cells
|
[>4] Siphonic type [C] Piercing type 95. Leydig cells produce [v4] Testosterone [C] Lutenizing hormone |
[>] Biting and chewing type [5] Oestrogen [.D] Epinephrine |
96. The estimated number of functional genes lli 111
[j4] 25,000 - 30,000 [S1 60,000 - 100,000
[C] 200,000 - 250,000 [D] 5,000 - 10,000
97. Identify the plant which is the most efficient converter of sunlight into chemical energy
iii ii uman beings
[?] Sugarcane [>] Green chillies
[B] Thiamin [I>] Nicotinamide
[J3] Nucleoside [D] A and C
[j4] Papaya [C] Tomato
98. Vitamin B2 is also called
[A] Riboflavin [C] Biotin
99. Adenosine triphosphate (ATP) is
[A] Nucleotide
[C] Nucleoside Triphosphate
100. All of the following compounds are intermediates of the Citric Acid Cycle EXCEPT
[A] Isocitrate [C] Succinate
[B] Malate [jD] Pyruvate
18
|
Attachment: |
| Earning: Approval pending. |
