University of Hyderabad (UoH) 2010 M.Sc Chemistry ENTRANCE - 2O1O - Question Paper
Booklet code C
Invigilators Signature
ENTRANCE EXAMINATION - 2010 M.Sc. Chemistry
TIME: 2 HOURS . VT1VjrTT14,
MAXIMUM MARKS: 100
HALL TICKET NUMBER: BOOKLET CODE:
1. Write your HALL TICKET NUMBER and the BOOKLET CODE in the space provided above and also in the OMR ANSWER SHEET given to you.
2 WOTk) nUmbered from 1 - 21 (excluding pages assigned for rough
3. There are 100 questions in this paper. All questions carry equal marks.
4. There is negative marking. Each wrong answer carries -0.33 mark.
5. Answers are to be marked on the OMR answer sheet following the instructions provided there upon.
6. Hand over both the question paper booklet and OMR answer sheet at the end of the examination.
7. In case of a tie, the marks obtained in the first 25 questions (PART A) will be used to determine the order of merit.
8. No additional sheets will be provided. Rough work can be done in the space provided at the end of the booklet.
9. Calculators are allowed.
10. Useful constants are provided on top of PART A in the question paper.
Rydberg constant = 109717 cm'1; Faraday constant = 96500 Cmol'1; Planck constant = 6.625 x 1034 Js; Speed of light = 2.998 x 108 ms'1; Boltzmann constant = 1.380 x 10'23 JK'1; Gas constant = 8.314 JK'Wl'1; Mass of electron = 9.109 x 1 O'31 kg; Mass of proton = 1.672 x 10'27 kg; Charge of electron = 1.6 x 10'19 C; 1 D = 3.336 x 1030 Cm; 1 bar = 105 Nm'2; RT/F = 0.059 V.
1. The dipole moment of BrF is 1.29 D, and its bond length is 178 pm. What is the percent ionic character of the Br-F bond?
(A) 3.9 (B) 33 (C) 8.5 (D) 15
2. The major product obtained in the following transformation is
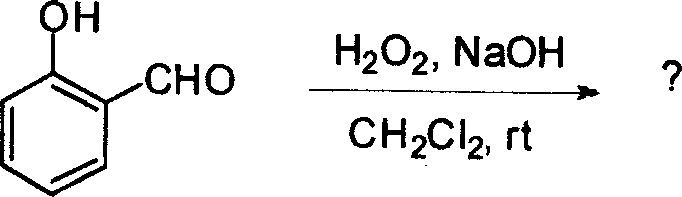
|
OH |
 |
(A)
(C)
(B)
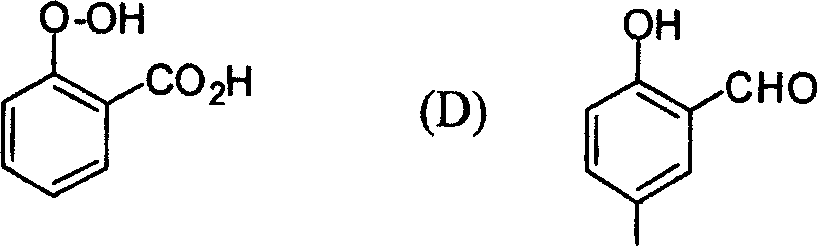 |
|
OH |
3. What is the charge required to make V(CO)6 an 18-electron species? (A) +1 (B) -1 (C) 0 (D) +2
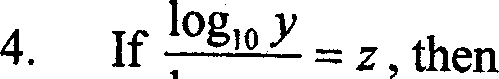 |
|
log10 * |
(A)logyx = z (B) logzx = y (C)logzy = x (D)logxy = z
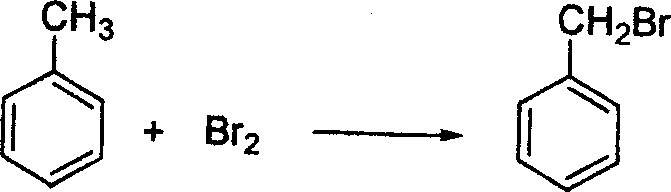
(A) nucleophilic substitution (B) electrophilic substitution
(C) free radical substitution (D) nucleophilic addition
6. which among the following is the strongest acid?
(A) Sulfuric acid
(B) Hydrobromic acid
(C) A solution of antimony pentafluoride in fluorosulfuric acid
(D) Fluorosulfuric acid
7. The critical solution temperature of water-phenol mixture at constant pressure is (A) invariant (B) bi-variant
(C) uni-variant (D) tri-variant
8. Which of the following functions changes its magnitude most rapidly at x = 0? (A)e'x (B)e 2x (C) (D) e
9. A capillary tube with an internal diameter of 0.2 mm is dipped into water. The surface tension of water is 73.6 dynes/cm. The height to which water rises in the capillary is (A) 10 cm (B) 100 cm (C)15cm (D)20cm
10. If the wave number of the O-H stretch vibration in the IR spectrum of CH3OH is 3300 cm , the wave number of the O-D stretch vibration of CH3OD is
(A) 3300 cm'1 (B) 2391 cm'1 (C) 2900 on1 (D) 1439 cm'1
following compounds is
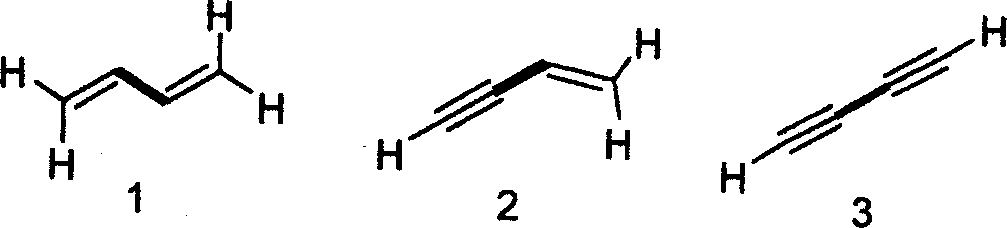 |
|
(A) 1>2>4>3 (B)3>1>2>4 (C)4>1>2>3 (D)2>1>3>4 |
H3C"CH3
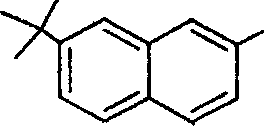
4
12. An aqueous solution containing NH4CI, FeCl3 and MnCl2 is treated with NH4OH solution. The observation is that
(A) both Fe3+ and Mn2+ will precipitate as hydroxides.
(B) only Fe3+ will precipitate as hydroxide.
(C) only Mn2+ will precipitate as hydroxide.
(D) NH4CI will crystallize out from the solution.
13. The curve that passes through the intersection point of the two lines x + y = 1 and y-x = l is
(A) y = x2 (B) y2 =x (C)x2-/=:1 (]D)x2+y2=l
14. A certain mass of gas occupies 5.50 liters at 300 K and 650 Torr. What will be its volume (liters) if it is cooled to 283 K and its pressure is increased to 980 Torr?
(A) 3.44 (B) 2.44 (C) 1.54 (D) 6.47
15. The locus of points equidistant from (3,0) and (5, 0) in the (X, Y) plane is (A) Y = 3 (B)X = 4 (C) Y = 4 (D)X = 3.
16. Among the following, which one is a crystallographic point defect?
(A) Edge dislocation (B) Screw dislocation
(C) Schottky defect (D) Stacking fault
17. An alkaloid contains C, H, N and O. Quantitative analysis of this compound showed the weight % of C, H and N to be, respectively, 70.8, 6.2, and 4.1. The empirical formula of the alkaloid is
(A)C7iH6ON4 (B) C40H42O8N2 (C) Ci4iHi202N8 (D) C20H21O4N
18. The number of elements expected in the g-block of the periodic table is (A) 14 (B) 18 .(C) 22 (D) 26
19. Which equation represents the following figure?
Ay
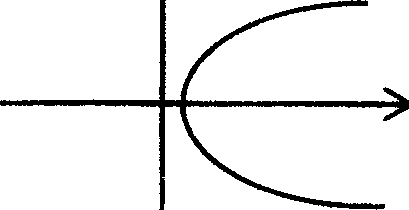
x
(A)x = 4y2 + 2 (B) y = 4x2 +2
(C)x = 3y2 (D)y = 3x2
20. Choose the correct statement regarding the relative stabilities of the enolates I and II.
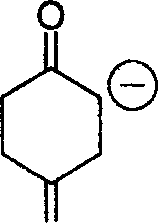
o
II
I
(A) I is less stable than II
(B) II is less stable than I
(C) I and II have same stability
(D) No comparison of stability can be made
21. Which of the following compounds undergoes hydroiodination to give roughly equal quantities of two products?
(A) 1-butene (B) 2-butene (C) 2-methyl-2-butene (D) 2-pentene
22. The curve y = x2 + x - 30 has a minimum at
(A)-6 (B) - 0.5 (C) 5 (D) - 30.
23. Which of the following is not suitable as an antacid?
(A) Sodium bicarbonate
(B) Calcium carbonate
(C) Sodium sulfate
(D) Magnesium hydroxide
24. You are given ten one-rupee coins and asked to make a close packing on the desktop. The highest coordination number that can be achieved in two dimensions is
(A) 4 (B) 6 (C) 8 (D) 9
25. Among Rb02, AIO2 , S1O2 and NC>2+, unpaired electron is present in (A)N02+ (B) A102 (C) Rb02 (D) Sr02
26. Which one among the given arrangements of one molecule of HC1 and one molecule of HBr will have the lowest energy?
|
H-Cl |
H |
-Cl |
|
(a) i ! |
(B) j | |
|
Br-H |
H |
-Br |
|
H- l 1 | ||
|
(C) H-Cl.......Br H |
(D) H |
ci |
|
'0 1 1 1> | ||
|
The determinant of the matrix |
10 0 0 |
is |
|
10 0 0 | ||
|
0 0 0 | ||
|
(A) 0 (B) 1 (C) 2 |
(D) 3 |
28. Crystals of an element possess a body-centered cubic (bcc) lattice structure. If the crystal undergoes a crystallographic transition to a face-centered cubic (fee) lattice, the unit cell length will change by a factor of
(A) V3/V2 (B) 1/V2 (C)V3 (D) V2/V3
29. An amino acid containing sulfur is
(A) proline (B) cystine (C) serine (D) alanine
HO OH
dil. H2SO4 ?
0-0
31. Application of the condition of exactness of the differential, dE - TdS - PdV, leads to (A)
| ||||||||||||||
|
~ U? |
yoy js \oojv
fdS} dP
32. For a molecule MX3 with zero dipole moment and M belonging to the second period, the orbitals on M involved in o-bonding are of the type
(A) pure p (B) sp hybrid (C) sp3 hybrid (D) sp2 hybrid
33. One mole of a perfect monatomic gas at 5.0 mbar pressure and occupying a volume of 1.66 m3 undergoes isobaric expansion to a volume of 16.6 m3. The work done by the gas (kJ) and the temperature change (degree) during expansion are, respectively,
(A) + 3.8, +1000 (B) + 7.5, + 900
(C) -3.8,- 1000 (D)-7.5,+ 900
:-V
(D) H3C
OCH3
O
O
o
|
(A) H3C | 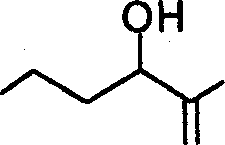 |
|
O | |
OH
|
OH | ||
|
H3C | 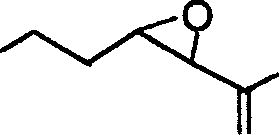 |
H |
(B) H3C
(C)
o
35. The equation r = O, where r is the distance of the point from the origin and <D is the angle of rotation from the x axis represents a
(B) monotonically decreasing function
(A) wave (C) circle
(D) spiral
36. Which of the following is responsible for global warming?
(A) UV radiation (B) Visible radiation (C) a-Radiation (D) Infrared radiation
37. In normal silicates, each silicon atom is surrounded by
(A) five oxygen atoms in a trigonal bipyramidal geometry
(B) four oxygen atoms in a square planar geometry
(C) four oxygen atoms in tetrahedral geometry
(D) six oxygen atoms in octahedral geometry
38. Consider the following equilibrium:
HA (aq) + H20 (/) H30+ (aq) + A'(aq)
Given the following acids and their equilibrium constants (Kc) at 25 C, which is the strongest acid?
(A) HF, Kc = 3.5 x 10-4 (B) HN02, Kc = 4.5 x lO4
(C) HOBr, Kc = 2.0 x 10'9 (D) HI03, Kc = 1.7 x 10'1
39. Given the relation f(2x) = 2f2(x) - 1, the value of f(x) for which f(2x) = f(x) is (A) 0 (B) 1/2 (C) 1 (D) 3/2
40. In the aluminothermit process, the function of aluminium is to act as
(A) a flux (B) a solder (C) a reducing agent (D) an oxidizing agent
41. Which one among the following set of arrangements is not feasible?
|
n |
/ |
m\ |
ms | |
|
(A) |
5 |
3 |
0 |
-y2 |
|
(B) |
4 |
2 |
-3 |
Vz |
|
(C) |
3 |
2 |
-2 |
Vi |
|
(D) |
3 |
0 |
0 |
y2 |
42. The metal present in Gilman reagent is
(A) Au (B) Ag (C) Zn (D)Cu
43. Decomposition of dinitrogen pentoxide is described by the chemical equation
2 N205 (g) 4 N02 (g) + 02 (g)
The rate of appearance of N02 is equal to 0.560 mol/min at a particular moment, what is the rate of appearance of 02 at that moment?
(A) 1.12 mol/min (B) 0.280 mol/min
(C) 0.140 mol/min (D) 2.24 mol/min
44. 14N, upon bombardment with a neutron gives ,4C + X. In another reaction, 14N upon bombardment with an a-particle gives ,70 +Y. X and Y are
(A) positron and neutron, respectively
(B) proton and neutron, respectively
(C) P-particle and proton, respectively
(D) both protons
45. The Newman projection and stereochemical structures of A and B are given below. The similarity between the two structures is that both
ch3
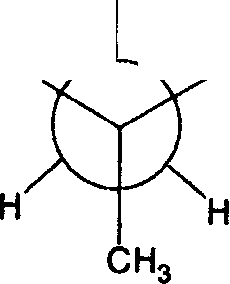
Hv / \ Br Br* -H
|
(A) are enantiomers (C) have the same cnirality S |
B (B) have the same chirality R (D) are aiastereomers |
46. Potassium crystallizes in a body centered cubic lattice with a = 5.20 A. The distance in A between the nearest neighbors, the number of nearest neighbors and the number of K atoms in the unit cell are, respectively,
(A) 3.69, 8 and 2
(B) 4.50, 8 and 2
(C) 4.50, 6 and 4
(D) 3.69, 8 and 9
47. The integrating factor of the equation (2x2 +y2 + x)dx + xydy = 0 is
(A)* (B) ex (C) log x (D)-
x
48. In acid medium, one mole of KMn04 will be equivalent to
(A) 2.0 moles of sulfite ion
(B) 2.5 moles of sulfite ion
(C) 5.0 moles of sulfite ion
(D) 0.4 moles of sulfite ion
49. Which of the following olefins will give the racemic alcohol after hydroboration reaction using B2H6 in THF followed by oxidation with H202/Na0H?
0
(A) Q
<Q O
(B)
(D)
o
50. One mole of an ideal gas expands isothermally from 1.0 liter to 10.0 liter at 300 K. The work done by the gas is
(A) 5757 J (B) 5102 J (C) 2501 J (D) 1250J
51. If f(x, y) = sin(ax2 + y), then is
dx
(A) cos ax2
(C) 2ax cos(ax2 + y)
(B) 2ax cos ax2 (D) cos(ax2 + y)
52. Choose the pair in which an effective acid base titration is not feasible.
(A) weak acid, strong base
(B) weak acid, weak base
(C) strong acid, strong base
(D) strong acid, weak base
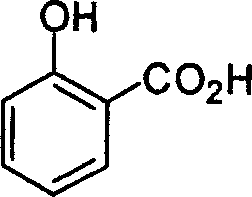
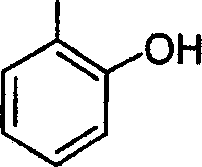
53. The major product obtained in the following transformation is
CH3
H3C'TV| och3 h3c Cl 0
AICI,
OCH,
 |
OCH, |
(D)
(B)
54. If a and p are the roots of the equation, 3x2 + 2x +1 = 0, then + is equal to
a P
(A)-1/3 (B) 2/3 (Ql+h/8 (D)-2
55. Consider the three processes: (i) sublimation of a solid, (ii) cooling a sample of Co(s) from 60 C to 25 C, and (iii) combustion of charcoal to form C02(g) and H20(g). The signs of AH and AS for the three processes (i), (ii), and (iii) are
(A) (i) AH>0 and AS>0; (ii) AH<0 and AS<0; (iii) AH<0 and AS>0
(B) (i) AH>0 and AS>0; (ii) AH<0 and AS>0; (iii) AH<0 and AS<0
(C) ((i) AH<0 and AS>0; (ii) AH<0 and AS<0; (iii) AH>0 and AS>0
(D) (i) AH<0 and AS<0; (ii) AH>0 and AS>0; (iii) AH<0 and AS>0
56. The net charge on the amino acid residue of aspartic acid at physiological pH is (A) +1 (B) 0 (C) -1 (D) -2
57. The number of lone pair of electrons around xenon in XeF3+, XeF4 and Xe03 are, respectively,
(A) 1,2 and 1
(B) 2,2 and 1
(C) 2, 1 and 2
(D) 1,2 and 2
58. A reaction follows the general rate law, Rate = k[A] [B]2. If the concentration of B is increased by a factor of 2, the rate of reaction will
(A) increase by a factor of 4 (B) increase by a factor of 2
(C) decrease by a factor of Vi (D) decrease by a factor of lA
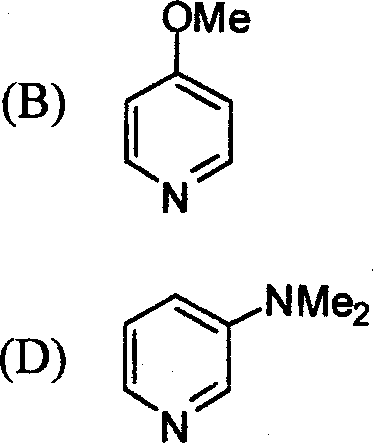
|
(A) |  |
|
N | |
|
NMe2 |
 |
|
N |
(C) I
60. The sum of the infinite series 1 + 2x + 3x2 + 4x3 +........is (|xl < 1)
(A) J- (B) (C) In* (D) e*
i-x - x)
61. If > = is
1 1 dx
(A) +1 for x > 0 and -1 for x < 0 (B) +1 for - oo < x < oo
(C) -1 for - oo < x < oo (D) -1 for x > 0 and +1 for x < 0
62. A supercritical fluid is a substance
(A) that is in the liquid crystal state
(B) with zero viscosity
(C) existing at a temperature and pressure above its Tc and Pc
(D) at its triple point
63. Among Ni(CO)4, [Ni(CN)4]2 and [NiCl4]2~,
(A) Ni(CO)4 and [NiCl4]2~ are planar while [Ni(CN)4]2 is tetrahedral
(B) [Ni(CN)4]2~ and [NiCl4]2 are planar while Ni(CO)4 is tetrahedral
(C) Ni(CO)4 and [Ni(CN)4]2~ are planar while [NiCl4]2- is tetrahedral
(D) [Ni(CN)4]2' is planar while Ni(CO)4 and [NiCl4]2~ are tetrahedral
64. The preferred conformation of the compound given below is
Mev/v ,OMe
Et'""" T)Me f 9Me OMe
(A) (B)
Et OMe OMe
Me .
(0) (0)
Et
65. According to the following half reaction, the molarity corresponding to a 0.4N solution of Na2Cr207 is
Cr2072~ + 6 e~+ 14 H+ 2 Cr3+ + 7 H20
(A) 0.4M (B)0.1M (C) 0.067M (D)2.4M
66. The complex number (1 + i)2 in polar form corresponds to (A) r = V2, 0 = tc/4 (B) r = 2, 0 = n/4
(C)r = V2, 0 = 3t/2 (D)r = 2, 0 = 72
67. Consider a relatively weak acid with pKa = 3.90. A base is added to this acid in order to obtain a buffer with pH = 4.12. Which of the following is closest to correct acid: base ratio in the buffer?
(A) 3.12 (B) 0.623 (C)3.1xio3 (D) 0.603
68. The line segments corresponding to 2x - 5y + 8 = 0 and x + 3y - 7 = 0 constitute the diameters of a circle of area 616 sq. units. The equation of the circle is
(A) (x-2)2 + (y-1)2 = 196 (B) (x-1)2 + (y-2)2 = 196
(C) (x-1)2 + (y-2)2 = 616 (D) (x-2)2 + (y-1)2 = 616
69. A condensation (step-growth) polymer among the following is
(A) Polystyrene
(B) Polyvinyl chloride
(C) Poly(ethylene terephthalate)
(D) Polypropylene
70. Which one of the following salts will be attracted most strongly by a magnet? (A) MnS04 (B) C0SO4 (C) ZnS04 (D) C11SO4
71. The proton NMR spectrum of 3 -pentanone shows
(A) one triplet and one quartet
(B) one singlet and one doublet
(C) one quartet and one doublet
(D) one singlet
72. The formal oxidation number of carbon in formaldehyde is
(A) -2
(B) 0 (C) 2 (D) 4
73. 72 g of ozone gas contained in a closed vessel at 1.0 atm pressure and 700 K temperature decomposes completely to oxygen gas. The pressure in the vessel changes to
(A) 0.67 atm (B) 1.0 atm (C) 1.5 atm (D) 2.0 atm
74. Three vectors, A, B, and C are defined as: A = 2i + 3 j - 4k, B = i - 2 j + 2k and
A A A
C = 3i-3j~k. Their vector triple product is given by (A) 31/ - 32] - 8k (B) 3 if - 32 j + 12k
(C) 30/-32/-8* (D) 31/-22j-Sk
75. Which one of the following ions does not interfere with the brown ring test for the nitrate ion?
(A)N02~ (B) Br~ (C)r (D)CF
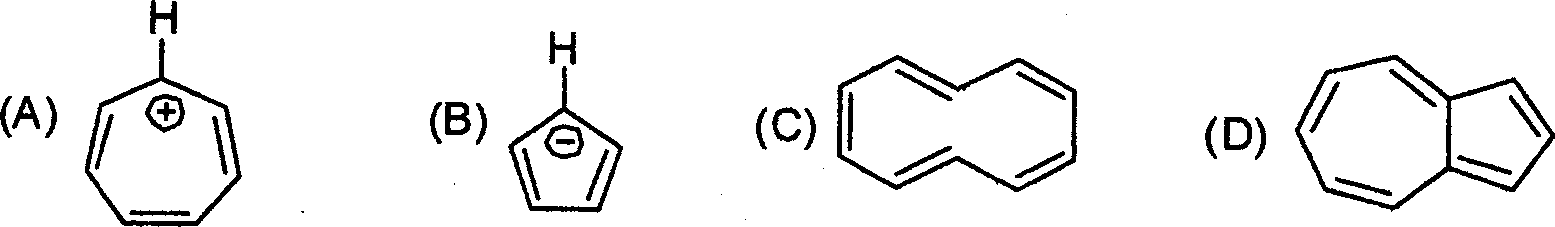
77. The carbonyl stretching band of ketones is usually observed around
(A) 1200 cm'1 (B) 1700 cm'1 (C) 2200 cm" (D) 3600 on*
78. The solubility product of AgBr at 25C is [E0AgBr(s)/Br- = 0.071 V and EAg+/Ag = 0.799 V at 25 C]
(A) 4.58 x 1013 (B) 6.28 x 10'7
(C) 9.12 x 102 (D) 4.58x 1015
79. The mole percentages of N2, O2, and Ar in dry air are, respectively, 78, 21 and 1. The average molecular weight of air is
(A) 29.0 (B) 92.0 (Q59.0 (D) 18.0
80. The characteristic violet color of biuret test is used to identify urea in a qualitative analysis. The structure of biuret is
(A) h2AAnh2 (B)
H H
VNY NH nh
(C) HN NH (D) A A
H2NNNH2
0
81. The soldering material used by electricians is an alloy of
(A) Cu and Pb (B) Zn and Cu (C) Sn and Pb (D) Fe and Zn
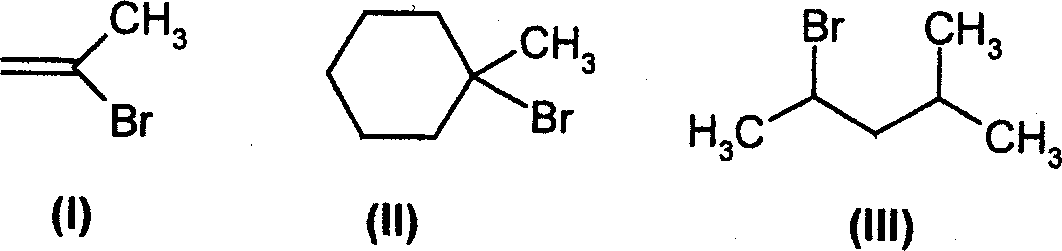
(A) II > I > III (B) I > II> III (C) II > III >1 (D) I > II > III
82. The order of decreasing rate of solvolysis with aqueous ethanol (fastest slowest) for the following bromides is
83. A three digit number divisible by 3 is to be formed using the numbers 0, 1, 2, and 3, without repetition. The total number of ways this can be done is
(A) 6 (B) 10 (C) 12 (D) 15
84. The correct order for increasing thermal stabilities among K2C03, MgC03, BeC03 and CaC03 is
(A) K2C03 < MgC03< CaC03 < BeC03
(B) BeC03 < MgC03< CaC03 < K2C03
(C) BeC03 < MgC03< K2C03 < CaC03
(D) MgC03 < CaC03 < BeC03 < K2C03
85. If a general point in the Cartesian coordinate system is represented by (x, y, z), and if a line is drawn from (0, 0,0) to (1,1,0) then what is the angle between this line and the z axis? (A) 0 (B) 45 (C) 90 (D) 180
86. The most probable outer electronic configuration for several lanthanides is given below.
La: 6s25d, Ce: 6s24?5d\ Pr: 6s24f3, Eu: 6s24f7, Gd: esVsd1,
Dy: 6s24f10, Er 6s24f12, Tm 6s24f13 Yb: Ssf14, Lu: 6s24f145d'
The elements that can have +2 as a stable oxidation state are
(A) Gd and Dy (B) Pr and Er (C) La and Lu (D) Eu and Yb
87. A fused five- and six-membered carbocyclic ring is an integral part of
(A) quinidine (B) cholesterol (C) a-pinene (D) chlorophyll
88. Bromine has a heat of vaporization of 30.91 kJmol'1 and its boiling point is 59 C. What is the entropy of vaporization of bromine?
(A) - 301 Jmor'K"1 (B) 10.7 JmolK1
(C) - 93.1 JmolK'1 (D) 93.1 JmolK*1
89. Choose the statement that is incorrect.
(A) Many Sn2 reactions are slowest in protic (hydroxylic) solvents.
(B) Sn2 reactions are the slowest in polar aprotic (nonhydroxylic) solvents
(C) SnI reactions are subject to large solvent effects.
(D) SnI reactions are favored by polar protic (hydroxylic) solvents.
90. A workshop contains 10 white, 5 black and 6 red cars to be repaired. If 4 cars are taken out at random, the probability that they consist of two white, one black and one red car is (A) 0.0251 (B) 0.0521 (C) 0.0125 (D) 0.0512
91. If 22 g of N2O5 reacts with 10 g of water to produce 22 g of nitric acid, the percentage yield of nitric acid is
(A) 32 (B) 69 (C) 87 (D) 100
92. If the vertices of a quadrilateral are A (0,0), B (0, 3), C (4, 3) and D (4, 0) then ABCD is
(A) a square (B) a parallelogram
(C) a rectangle (D) a trapezoid
93. If sum of a series is defined as, Sn = 1 + 2 + 4 + 8 + 16 +.......+ 2n, the value of S20 is:
(A) 221 -1 (B)220 (C) 20! (D)220 + 202
CH3OH
_/C02CH3 CH2OH
(A)
(B) N
OH .CHO
ci
(C)
|
N |
 |
|
OH |
Ph
O
0 if'
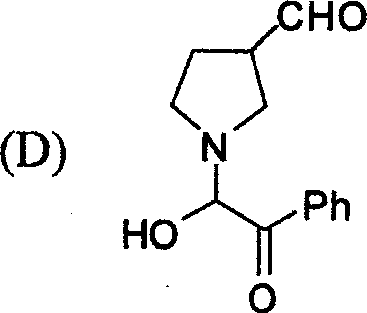
95. The boiling point of 0.1 M glucose solution under 1 atm pressure is 100.02 C. The boiling point of 0.25 M K2SO4 solution under 1 atm pressure would be
(A) 100.5 C (B) 100.6 C (C) 101.4 C (D) 100.15 C
96. Two van der Waals gases (A and B) have the same values of a but different values of b. The correct statement among the following is
(A) Both are equally compressible
(B) Gas with lower b value is more compressible
(C) Gas with higher b value is more compressible
(D) Neither A nor B is compressible.
97. Liquids A and B form an ideal solution at all mixing ratios. At 50 C, a solution containing
1 mole of A and 2 moles of B has a total vapor pressure of 110 mm Hg. When 1 mole of A is added to this solution, the total vapor pressure changed to 105 mm Hg. If 1 mole of B is now added to this solution containing 2 moles of A and 2 moles of B, the total vapor pressure (in mm Hg) will be
(A) 106 (B) 108 (C) 110 (D) 112
98. Which.change inthesystemwill
fe) no2(g) +N03(g)
(A) an increase in the amount of N->n<r /m ,
cunuuni or JN2U5 (B) a decrease in the amount of N03
(C) an increase in pressure /tvv fln :n.m!K,0 ,
\u) an increase m volume.
For the reaction 2HI - H2 +I2, the values of rate constants are 1.2 x 103 and 3 0 x 1 O'5
Z r" 7 K 4 629 K The of the reaction is
( ) 0.0 kcalmol (B) 54.0 kcalmol" (C) 45.5 kcalmor1 (D) 54.5 kcalmol'1
100. lie valence electronic configuration of two atoms with atomic numbers Z, and Z2 are 3s 3p and 4s 4p5, respectively. The difference Z2 - Z, is
<A) 8 W 10 (C) 18 (D) 20
|
Attachment: |
| Earning: Approval pending. |
