University of Hyderabad (UoH) 2011 M.Sc Chemistry Entrance model - Question Paper
UNIVERSITY OF HYDERABAD ENTRANCE EXAMINATION - Chemistry Model Questions
M. Sc. Chemistry
TIME: 2 HOURS MAXIMUM MARKS: 100
Useful Constants:
Rydberg constant = 109737 cm -1; Faraday constant = 96500 C; Planck constant = 6.625 x 10"34 J s; Speed of light = 2.998 x 108 m s-1; Boltzmann constant = 1.380 x 10"23 J K-1; Gas constant = 8.314 J K"1mol"1; Mass of electron = 9.109 x 10-31 kg; Mass of proton = 1.672 x 10-27 kg; Charge of electron = 1.6 x 10-19 C
PART - A
1. The reaction of 10.23 g of Fe2O3 with excess carbon, Fe2O3 + 3C 2Fe + 3CO yields 8.94 g of Fe. What is the percentage of yield? (At. wts: Fe = 55.05, C = 12.01, O = 16.00) (A) 78% (B) 84% (C) 80 % (D) 76%
2. A normal to the surface x2yz + 3y2 - 2xz2 + 8z = 0 at the point (1, 2, -1) is
(A) i + 2 j - k (B) - 6i +11 j + 14k
(C) 3i +11 j + 14k (D) 11i - 6 j + 14k
3. How many electrons are transferred in the following reaction?
2Zn(s) + Ag2O2(s) + 2H2O(l) + 4OH"(aq) - 2Ag(s) + 2Zn(OH)42-(aq)
(A) 4 (B) 6 (C) 2 (D) 3
4. The difference in energy between the axial and the equatorial conformations of -butylcyclohexane is
(A) 1 kcal/mol (B) 20 kcal/mol (C) 15 kcal/mol (D) 6 kcal/mol
5. The maximum number of phases that can coexist in equilibrium in a binary system is
(A) 4 (B) 3 (C) 2 (D) 0
6. The points A (1, -1), B (3, 2) and C (7, 8) form
|
(A) An equilateral triangle (C) A curve |
(B) An isosceles triangle (D) A straight line |
7. A hydrate of nickel bromide has the formula NiBr2-xH2O. 18.2 g of a sample of this hydrate is heated to a constant weight of 14.6 g. The value of x is (At. wts: Ni = 58.7, Br = 79.9,
O = 16.0, H = 1.0)
(A) 6
(B) 3
(C) 1
(D) 4
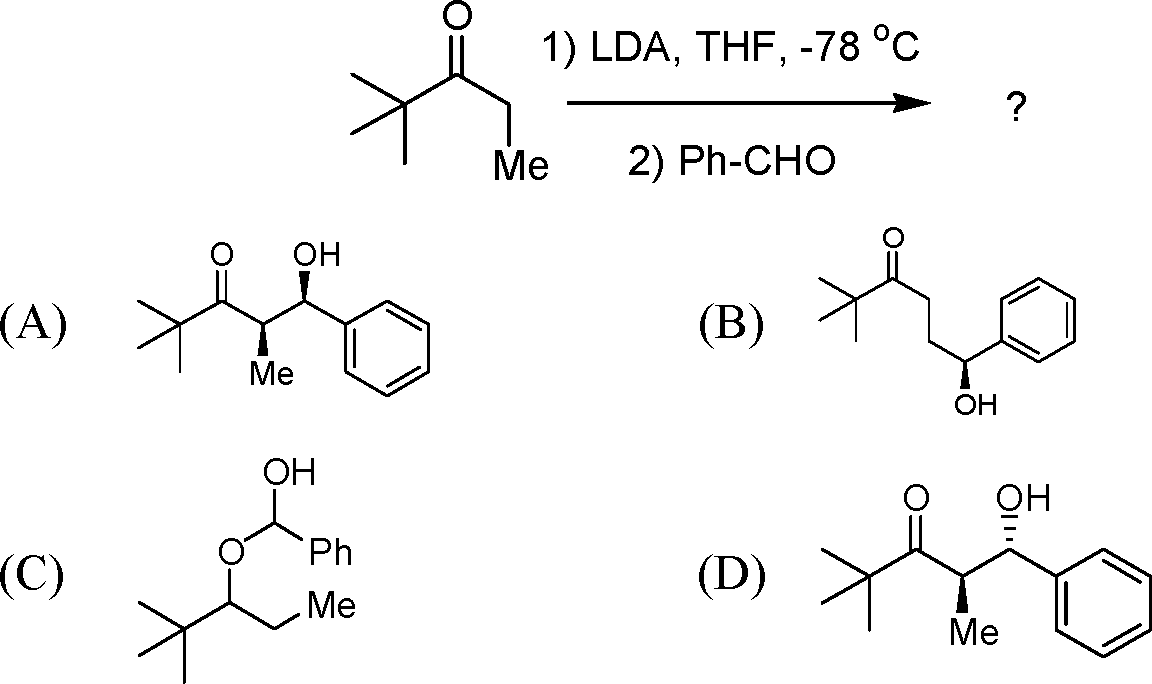
8. The enol content of CH3COCH2CO2Et in hexane is 46% and that in water is 0.4%. The reason for the above observation is as follows:
(A) Intermolecular hydrogen bonding is stabilized by hexane.
(B) Intramolecular hydrogen bonding is stabilized by water.
(C) Intramolecular hydrogen bonding is destabilized by water.
(D) CH3COCH2CO2Et dissolves in hexane completely.
9. A three-fold increase of the pressure affects the yield of the following reaction n2 (g) + 3 h2 (g) w 2 nh3 (g) at equilibrium by a
(A) nine-fold increase. (B) six-fold increase.
(C) nine-fold decrease. (D) three-fold increase.
10. The graph of 4x2 - 9y2 -16x + 18y - 29 = 0 represents
(A) a parabola (B) a hyperbola (C) an ellipse (D) a circle
11. Structure of carbon suboxide (C3O2) is
(A) tetrahedron (B) bent (C) trigonal pyramid (D) linear
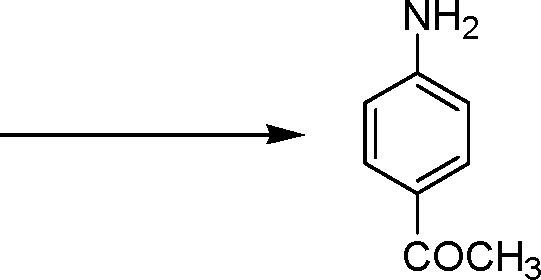
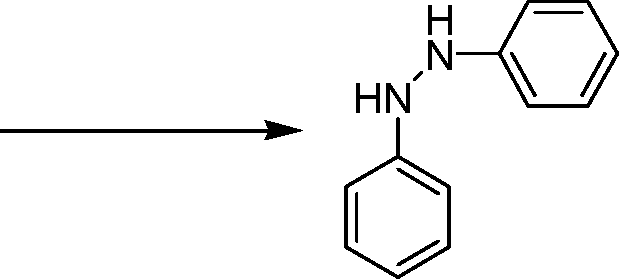
12. Identify Sandmeyer reaction from the following
|
nh, | |
|
(A) |  |
|
nh, | |
 |
Br |
|
nh, |
 |
(B)
|
NH, |
 |
|
NH, |
 |
(C)
|
CN |
 |
13. The nearest cation-anion distance in a crystal which adopts the NaCl (rocksalt) structure is 2.5 A. The nearest cation-cation distance is
(A) equal to the nearest anion-anion distance. (B) 3.54 A.
(C) j= where a is the unit cell length. V2
(D) all of the above.
7x9 - 4x5 + 2x -13
lim-38-2-=
x- 3x + x - 5x + 2x
(A) - 7/3 (B) 0 (C) (D) - 13/2
15. High thermal stabilities of transition metal carbonyls is due to
(A) non availability of d-orbital on carbon.
(B) formation of ionic bond between CO and metal.
(C) interaction of filled metal d-orbital with the empty antibonding n* orbital of CO.
(D) covalent bonds of oxygen in CO with antibonding n* orbital of metals.
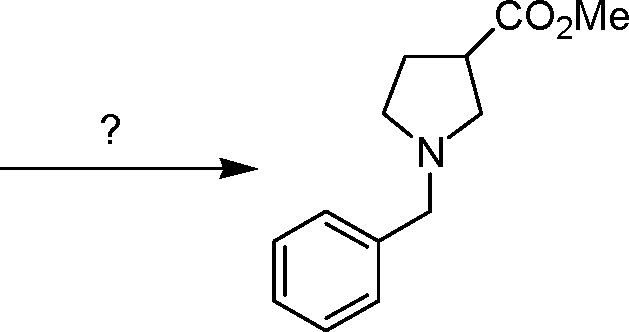
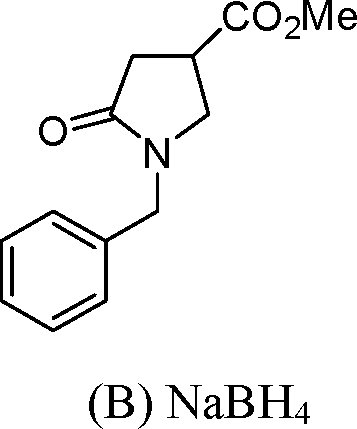
16. The intermediate involved in the identification of glucose in Molisch test is JD. XHO OHCOCHO
(A) QT (B) LJ
(C) HO[TOH (D) HO[fjfCHO
17. Which is the thermodynamically most stable allotrope (or polymorph) of carbon?
(A) Diamond (B) Hexagonal diamond
(C) Buckminsterfullerene or C60 (D) Graphite
18. The global maximum of the function, f (x) = e x .cosx , occurs at x =
(A) 3n (B) n (C) 2n (D) 0
19. The photosynthetic process in green plants consists of
(A) splitting of elements of water, followed by oxidation of oxygen to ozone.
(B) splitting of elements of water, followed by reduction of carbon dioxide.
(C) reaction of water with carbon dioxide.
(D) reaction of water with oxygen.
20. The sp hybridized carbocation among the following is
H

(A) (B) A

I
(C) J (D)
O
21. 20 mL of 0.2 M hydrochloric acid is added to 5 mL of 0.1 M sodium carbonate. The resultant solution is then titrated against 0.2 M sodium hydroxide. What will be the titre value?
(A) 15 mL (B) 10 mL (C) 5 mL (D) 20 mL
2
22. If f (x) =-, nth derivative off (x) is
1 - x
(A) 2(n)(1 - x)-(+1) (B) 2(n!)(1 - x)( n+1)
(C) 2(yfn )(1 - x)(n+1) (D) 2(n!)(1 - x)-(n+1)
23. The number of lone pair(s) in XeOF4 is/are
(A) 0 (B) 3 (C) 2
24. Nef reaction is the conversion of
|
(A) |
no2 A |
n'oh . .A. |
(B) |
no2 A |
nh2 -- A |
|
(C) |
no2 A |
OH |
(D) |
no2 A |
O -- A |
25. The second ionization potential of three successive elements in the periodic table are 2856, 3388 and 3374 kJ/mole, respectively. These elements are likely to be
(A) C, N, O (B) O, F, Ne
(C) N, O, F (D) F, Ne, Na
PART -B
30
dx
It
26.
_- + 4 xz (A) n
3n
2
n
2
n
4
(B)
(C)
(D)
27. Neil Bartletts motivation to study Xe compounds (Noble gases) came from one of the following observation.
(A) Ability of O2 to react with metals to form dioxygen complexes.
(B) PtF6 can oxidize O2 to form a crystalline orange-red solid.
(C) Ability of O2 to react with halogens to form halogen oxides.
(D) The abundance of O2 on earths crust.
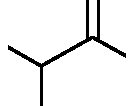
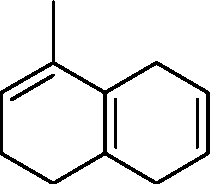 |
|
triene |
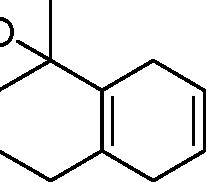
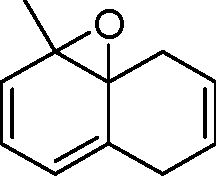
(A)
(B)
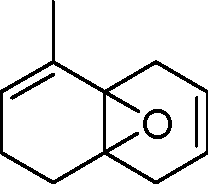
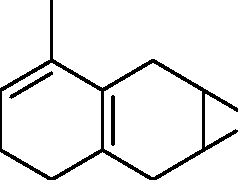 |
O |
(C)
(D)
29. The (100) plane of a simple cubic crystal diffracts at 60 . The angle at which the (111) plane would diffract is
(A) 75 (B) 45 (C) 60 (D) 30
30. Number of ways a committee consisting of 2 mathematicians and 3 chemists can be formed out of total 5 mathematicians and 7 chemists is
(A) 530 (B) 350 (C) 450 (D) 540
31. Which sulphide is not precipitated from acidified aqueous solutions by hydrogen sulphide? (A) MnS (B) PbS (C) CuS (D) AgS
32. Which one of the following reagents reacts with an aldehyde and produces a trans olefin as a major isomer?
(A) PhsP=CH2 (B) PhsP=CHOCH3 (C) PhsP=CHCO2CHs (D) PhsP=CHCHs
33. 20 mL each of 0.3 M NaOH and 0.1 M HCl are mixed together. The pH of the resulting solution is
(A) 14 (B) 0 (C) 1 (D) 13
34. The diagonals are perpendicular bisectors to each other for
(A) rhombus and square only.
(B) parallelogram and rectangle only.
(C) parallelogram, rectangle, rhombus and square.
(D) rectangle and square only.
35. In an oxide of element E, two-thirds of E is in +3 oxidation state and the remaining in +2 oxidation state. What would be the formula of this oxide?
(A) EO (B) E2O5 (C) E3O4 (D) E2O3
36. The enthalpy change associated with the following hydrogenation is
ch3ch=chch3 + h2 - ch3ch2ch2ch3
(Given data: C-H = 98.2 kcal/mol; C-C = 80.5 kcal/mol; H-H = 103.2 kcal/mol C=C = 142 kcal/mol)
(A) 28.7 kcal/mol (B) -28.7 kcal/mol (C) 157.7 kcal/mol (D) -157.7 kcal/mol
37. 0.53 g of anhydrous sodium carbonate was dissolved in a concentrated HCl solution in an open container. The work done by the released carbon dioxide is
(A) 1.1 lit. atm. (B) 0.23 lit. atm. (C) 0.11 lit. atm. (D) 2.3 lit. atm.
|
38. In the following right angled triangle the distances CD (x) and AC (y) are C | |
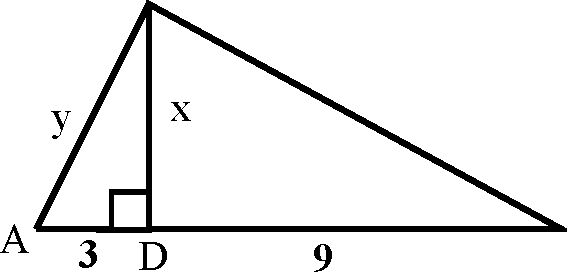 |
B |
(B) x = 3V3, y = 6 (D) x = 3V3, y = 9
(A) x = 6, y = 9 (C) x = 9, y = 3V3
39. Which of the following compounds is most reactive with ozone?
(A) carbon dioxide (B) sulphur dioxide (C) nitrogen (D) hydrogen fluoride
H3C ch3
3 + b2H6
H3C CH3
h3c ch3\ 3yB
H3C C-3/
h3c ch3
3m 3
h3c ch3
(A)
(B)
|
CH3 HC | |
|
h3c | 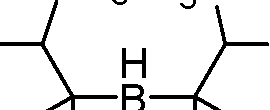 |
|
-3C I,, 1 , CH3 3 ch3 ch3 3 | |
c
(C)
(D)
H3C ch3 3-H2 h3c ch3
41. The gas with the largest van der Waals a coefficient among the following is (A) CH4 (B) NH3 (C) H2O (D) BH3
x
42. What is the second derivative of x +--?
2
(A) x
(B) 1
(D) 0
(C) x + 1
43. Which of the following radicals causes depletion of ozone and oxygen in the stratosphere? (A) NO2 (B) ClO (C) NO (D) CO
44. The reagent(s) required for the following transformation is
H D
Ph D -
Ph H
(A) H2, Pd/BaSO4 (B)H2, Pd/C (C) Na/liq. NH3 (D) NaBH
45. The molecular formula of glucose is C6H12O6. The chemical composition of glucose is
(A) 39.99 % C, 6.71 % H, 53.27 % O (B) 40.99 % C, 6.71 % H, 53.27 % O (C) 39.99 % C, 7.71 % H, 54.27 % O (D) 29.99 % C, 7.71 % H, 53.00 % O
(A) 1 (B) x (C) x3 (D) x2
47. Which of the following ions present in NaCl, KCl and CaCl2 has a larger ionic radius compared to the corresponding neutral atom?
(A) Cl- (B) Ca2+ (C) K+ (D) Na+
48. The most appropriate reagent required for the Michael addition to cyclohex-2-enone is (A) MeLi (B) MeMgBr (C) Me2CuLi (D) MeMgCl
49. A mercury filled manometer is connected to a gas cylinder with the other end open to the atmosphere. If the level of mercury in the arm connected to the cylinder is 24.7 cm higher than that in the open arm and the atmospheric pressure is 0.975 atm, the pressure (in atm) in the gas cylinder is
(A) 0.45 (B) 1.65 (C) 0.65 (D) 0.25
50. Given that A and B are two sets; the correct statement among the following is
(A) AIB c A U B (B) A U B c AIB
(C) A - (A IB) c AIB (D) (A - B) c AIB
51. Which of the following is not true for superacids?
(A) They are considerably more basic than normal concentrated acids.
(B) They possess highly negative pH.
(C) They are non-aqueous acids.
(D) They are aqueous acids.
OH
(A) 1-Penten-3-one (B) (Z)-2-Penten-4-one
(C) ()-3-Penten-2-one (D) ()-2-Penten-4-one
53. Gases W, X, Y and Z obey van der Waals gas equation with a and b (in suitable units) values as given in the table below
|
W |
X |
Y |
Z | |
|
a |
6 |
6 |
20 |
0.05 |
|
b |
0.025 |
0.15 |
0.10 |
0.02 |
Which of the gases have (i) the highest critical temperature, (ii) the largest molecular volume and (iii) the most ideal behavior at STP?
(A) W, X and Z respectively (B) W, X and Y respectively
(C) Y, Z and W respectively (D) W, Z and X respectively
54. What could be the Cartesian coordinates of the point which when joined to the origin will give a line lying on the xy plane?
(A) (1,1,1) (B) (1,1,0) (C) (0,0,1) (D) (0,1,1)
55. Chlorophyll contains the following metal ion:
(A) Ni2+ (B) Co2+ (C) Fe2+ (D) Mg2+
56. Identify the a-D-glucose from the following:
(B) HOOH
|
HOH | |
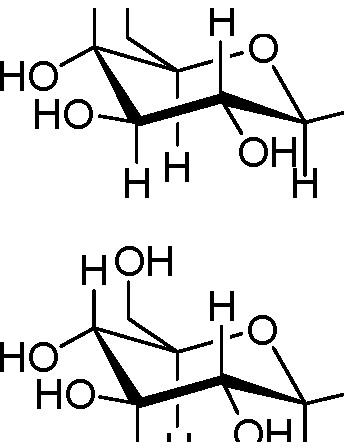 |
OH H |
|
HH oh0h | |
(A)
(C)
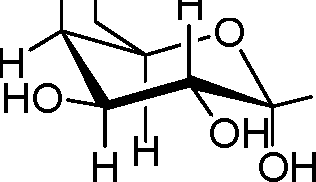 |
H |
(D)
|
HOH | |
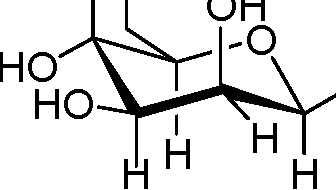 |
OH |
57. Blood is said to be isotonic with 0.85% (w/v) NaCl solution at 40 C. Assuming the complete dissociation of NaCl what will be the freezing point of blood? [Kf (water) = 1.86]
(A) - 0.054 C (B) - 0.54 C (C) 0.54C (D) - 0.154 C
58. The monotonically increasing function among the following is (A) tanhx (B) sinhx (C) tan x
(D) sin x
59. Which of the following contains metal-metal bond?
(D) Fe2(SO4)3
(A) Na2SO4 (B) Al2(SO4)3 (C) Hg2SO4
60. The rate of SN1 reaction (in ethanol) of the following substrates decreases in the order of
,OTs
EtOTs
IV
II
(B)
(D)
I
(A) III > IV > I > II (C) IV > III > II > I
PhCH2OTs
CH3OTs
III
II > I > IV > III I > II > IV > III
61. A spectrophotometer cell when filled with liquid X transmits 50% and when filled with another liquid Y transmits only 25% of the incident light of a certain wavelength. What would be the optical density at this wavelength when the same cell is filled with a mixture of equal volumes of the two liquids?
(A) 0.60 (B) 0.45 (C) 0.30 (D) 1.00
2t
62. A particle of unit mass experiences a force of 10e . The velocity of the particle after infinitely long time would be
(A) 5 (B) 20 (C) 10 (D)
63. A mixture of three volumes of conc. HCl and one volume of conc. HNO3 is known as aqua regia. It contains
(A) free NO3- and HClO4 and hence is a powerful oxidizing agent.
(B) free Cl2 and ClNO and hence is a powerful reducing agent.
(C) free Cl2 and ClNO and hence is a powerful oxidizing agent.
64. The isoprene rule may be used to derive the bio-synthetic pathway for the natural product
(A) D-Glucose (B) Caffeine (C) Atropine (D) Geraniol
65. Calculate E0 for the process M M3+ + 3e. Given E0 = 0.44 V for M M2+ + 2e and E0 = -0.77 V for M2+ M3+ + e.
(A) 0.037 V (B) 0.563 V (C) 0.850 V (D) 0.331 V
66. The minimum and maximum number of points at which a pair of straight lines and an ellipse intersect in a plane would be respectively
(A) 0, 2 (B) 0, 4 (C) 2, 2 (D) 2, 4
67. Cooling a solution of sodium in ethyl amine in the presence of 2,2,2-crypt, results in the formation of
(A) NaNH2 complex with 2,2,2-crypt (B) NaNH2
(C) Na(HNCH2CH3) (D) [Na(2,2,2-crypt)]+Na-
68. The order of decreasing basicity of the following compounds is
N 'N' v 'N
H N H
I II III IV
(A) II > III > I > IV (B) III > II > I > IV
(C) IV > I > II > III (D) IV > I > III > II
69. Given that Kb = 1.8 x 10-5 M, the pH of a 0.1 M NH3 solution is closest to
(A) 9 (B) 11 (C) 13 (D) 10
70. The sum of the cubes of the first n natural numbers is
n 3(n3 +1) n 3(n +1)3 n 2(n +1)2 n 2(n2 +1)
3 8 4 4
71. Which of the following is an example of an organometallic compound?
(A) CHsMgBr (B) Na2CO3 (C) NaOOC-COONa (D) (CHb)2-C=C-(CHb)2
72. The most favorable product obtained in the following reaction is
|
O |
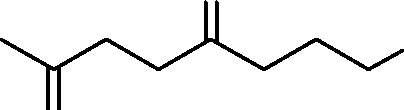 |
|
O |
|
KOH CO2H - ? 2 A O |
 |
|
O | ||
|
(A) |  |
co2h |
|
O | |
|
(C) |  |
co2h
73. The concentration of Ag+ ion in a saturated solution of Ag2C2O4 is 2.3
10-4 mol/L. The
X
solubility product of Ag2C2O4 is
-8
12
(A) ~5.29 x 10
(B) ~6.00 x 10' (D) ~1.15 x 10
-12
-4
(C) ~12.17 x 10
74. If xy = constant, =
dx
(B) - y
y
y-1
(D) yx
(A) 0
(C) -
x ln x
x
75. Which of the following contains a three-centre two electron bond?
(A) B2H6 (B) N2H4 (C) C2H6 (D) O2F2
76. The most appropriate reagent(s) for the following conversion is
 |
|
(C) Zn(Hg)/HCl |
(A) LiAlH4
(D) BHs-THF
77. Consider a crystal having face centered cubic lattice structure made up of atoms having a radius of 1.414 A. Assuming that 4 x 106 atoms form a spherical crystalline particle, the radius of the particle in A is
(A)112 (B)248 (C)224 (D)312
|
78. A segment of a circle is shown below with some of the lengths marked on it. The radius of the circle is C |
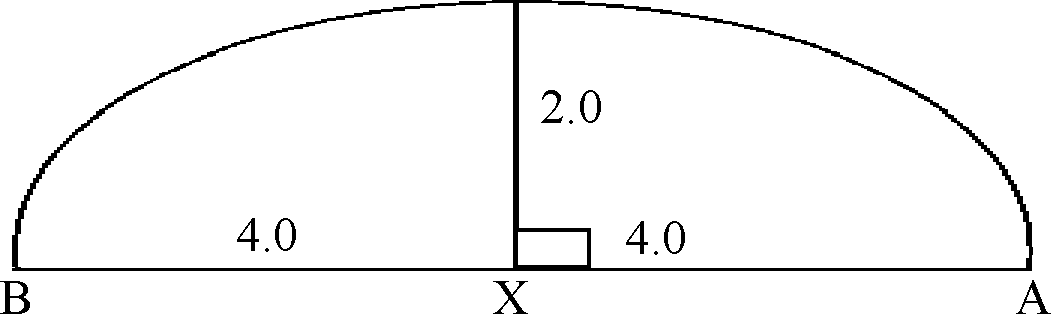 |
|
(B) 5.5 (C) 5.0 |
(A) 6.0
(D) 4.0
79. Identify the metal containing pigment in the prosthetic group of hemoglobin.
(A) Iron porphyrin (B) Zinc porphyrin
(C) Copper porphyrin (D) Cobalt porphyrin
rBuOK
|
BuOH O O | |
|
Cl | 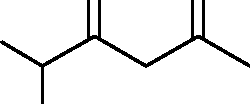 |
|
ch3 | |
CO2Et
(B)
80. The product obtained in the following reaction is
O
C U
(A)
O
O
|
+ |  |
OEt |
|
CH3 | ||
O COMe
|
rBuO | 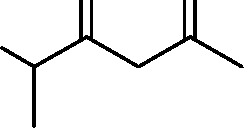 |
(C)
(D)
EtO2C Me
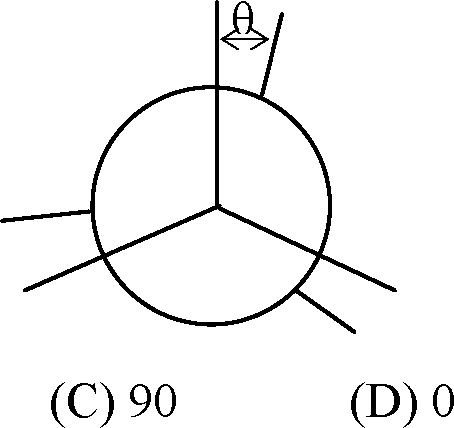
81. The conformations obtained by rotation about the carbon-carbon single bond in ethane can be distinguished by the angle, 9 shown in the figure below. For which value of 9, will the structure be chiral?
82. The faces of a cube are painted with three colors red, green and blue so that the opposite faces have the same color. If three such cubes are rolled what is the probability of getting three different colors on the top faces?
(A) 5/36 (B) 6/27 (C) 1/8 (D) 1/9
83. A mixture of Al(OH)3 and Fe(OH)3 can be separated by treatment with a solution of (A) HCl (B) CH3COOH (C) CH3OH (D) NaOH
84. The infrared absorption most affected by intra-molecular hydrogen bonding in 2-hydroxy acetophenone is
(A) methyl group C-H stretching. (B) hydroxyl group O-H stretching.
(C) aromatic ring C-H bending. (D) aromatic ring C-C stretching.
85. 12.8 g of an ideal gas occupies 10 L at a pressure of 750 mm of Hg and at 27 C. The molecular weight of the gas is
(A) 33.2 (B) 30.3 (C) 31.9 (D) 34.7
86. According to valence bond theory the hybridization and the number of unpaired electrons in octahedral [Mn(CN)6]4- are
(A) d2sp3 and one unpaired electron.
(B) d2sp3 and zero unpaired electron.
(C) sp3d2 and five unpaired electrons.
(D) sp3d2 and zero unpaired electron
87. The major product obtained in the following reaction is
O
MeO
A
OEt
+
MeO.
 |
|
CO,Me MeO |
MeO.
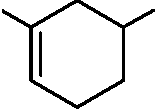
(B)
(D) kX
CO,Me
MeO,C vsx'',",Xss
(o xx
CO,Me
(A)
OMe
88. The average kinetic energy of a molecule of H2 at 0 C is
(A) 5.7 x 10-14 ergs
(B) 3.4 x 1010 ergs
(D) 2.3 x 1014 ergs
(C) 4.8 x 10-10 ergs
83. The element having the electronic configuration 1s22s22p63s23p64s23d2 is (A) Sc (B) Ti (C) Cr (D) V
30. The major product obtained in the following reaction is
91. The rate constant of a first order reaction is 3.83 x 10 3 s 1. If the initial concentration of the reactant is 0.60 M, what will be the concentration after 142 s?
(A) 0.463 M (B) 0.432 M (C) 0.348 M (D) 0.400 M
 |
NH2 |
(D)
h2n-
-NH'
92. Electrolysis of brine produces
(A) hydrogen and chlorine.
(B) sodium hydroxide, hydrogen and chlorine.
(C) sodium, hydrogen and chlorine.
(D) sodium hydroxide and chlorine.
93. The final product obtained in the following transformation is
1) HCl/H2O N2 1) Sn/HCl 2) NaNO2
- - ?
2) NaH/H2O 3) Ph-OH
(A) N=N-OH (B) HNOH
(C) HO
94. In thermodynamics, which of the following terms stands for the heat absorbed by a system held at constant pressure?
(A) Enthalpy (B) Entropy
(C) Free energy (D) Heat capacity
95. Which among the following three isobars 'Cd, "In and "Sn is/are likely to be radioactive?
(A) Cd (B) 114Sn and In (C) Sn (D) In
96. The best method for the preparation of (CH3)3C-O-CH3 is
(A) (CH3)3C-OH + CH3I catalyzed by H2SO4 (B) (CH3)3C-OK + CH3I
(C) (CH3)3C-H + CH3OH catalyzed by H2SO4 (D) (CH3)3C-Cl + NaOCH
97. The compounds H3PO2, H4P2O7, HPO3 and H3PO4 are respectively
(A) hypo, pyro, meta and ortho acids.
(B) meta, pyro, hypo and ortho acids.
(C) ortho, pyro, meta and hypo acids.
(D) hypo, ortho, meta and pyro acids.
98. The product obtained in the following transformation is
,CHO
O O
+
O
x
h2n nh2
90 oC
1 h
+
H3C
|
O |
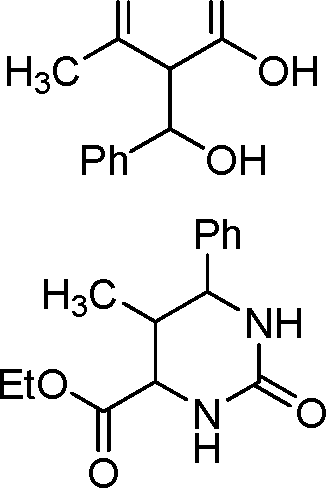 |
nA
o
A
A X
H3CVs'/T>H
(A)
(B)
O Ph
AA
h3c no
EtO'
NH
(C)
(D)
H
99. The absolute configuration of the chiral centers 1 and 2 in the following molecule are
OH
H3C 1 ch3 Ph
(A) (1S, 2S)
(B) (1S, 2R)
(D) (1R, 2S)
(C) (1R, 2R)
100. Given that the pure gold is 24 Karat, the composition of the alloy Cu3Au in Karat is [At. Wts.: Cu ~64, Au ~197]
(A) ~12 (B) ~22 (C) ~18 (D) ~20
22
|
Attachment: |
| Earning: Approval pending. |
