University of Hyderabad (UoH) 2011 M.Sc Chemistry Entrance for - exam paper
ENTRANCE EXAMINATION - 2011 M. Sc. Chemistry
MAXIMUM MARKS: 100
TIME: 2 HOURS
HALL TICKET NUMBER:
BOOKLET CODE:
1. Write your HALL TICKET NUMBER and the BOOKLET CODE in the space provided above and also in the OMR ANSWER SHEET given to you.
2. Make sure that pages numbered from 1 - 18 are present (excluding pages assigned for rough work).
3. There are 100 questions in this paper. All questions carry equal marks.
4. There is negative marking. Each wrong answer carries - 0.33 mark
5. Answers arc to be marked on the OMR answer sheet following the instructions provided there upon.
6. Hand over both the question paper booklet and OMR answer sheet at the end of the examination.
7. In case of a tie, the marks obtained in the first 25 questions (PART-A) will be used to determine the order of merit.
8. No additional sheets will be provided. Rough work can be done in the space provided at the end of the booklet.
9. Only non-programmable calculators are allowed.
10. Useful constants are provided on top of PART-A in the question paper.
I
Useful Constants:
Rydberg constant = 109737 cm"1; Faraday constant = 96500 C; Planck constant = 6.625 x 10'34 J s; Speed of light = 2.998 x 108 m s'1; Boltzmann constant = 1.380 x 1023 J K1; Gas constant = 8.314 J Kmol'1; Mass of electron = 9.109 x 1031 kg; Mass of proton = 1.672 x 10'27 kg; Charge of electron = 1.6 x 1019 C; 1 D = 3.336 x 10'30 C m; 1 bar = 10s N m"2; RT/F = 0.059 V
1. Which of the following is not a crystalline substance?
(A) Charcoal (B) Graphite (C) Diamond (D) Cm
2. The major product expected from the following reaction is
h2o
H2S04
OH OH
(B) H30'ACh3
3. Consider the equilibrium 2Y with equilibrium constant, Kc = 3.6 M at 25 C. If the initial concentrations are [X]o = 1.0 M and (Y]o = 0.0 M, the equilibrium concentration of X at 25*C, [XJcq is
(A) 0.33 M (B) 0.36 M (C) 0.40 M (D) 0.60 M
4. The sides of a triangle arc of length 3.0,4.0 and 5.0 cm. If the side with length 5.0 cm is the base, what is the height of the triangle?
(A) 2.4 cm (B) 2.8 cm (C) 3.4 cm (D) 4.0 cm
5. Which one among the following chlorides is dissociated to the least extent in aqueous solution?
(A) ZnCli (B) HgCl2 (C) BaCl2 (D) A1C13
6. The IUPAC name for the following compound is
/= CH2
h3c <
CH3
(A) 4-vinyl-2-pentyne (B) 4-methylhex-2-yn-5-ene
(C) 3-methylhex-4-yn-l-ene (D) 3-mcthylhcx-l-en-4-yne
7. X-ray diffraction study of a crystal with a simple cubic lattice structure shows diffraction from the (110) plane appearing at the Bragg angle 6 = 20. The angle at which the diffraction from the (220) plane will appear is
(A) 9.8 (B) 10 (C)40 (D) 43
8. Consider the plot of the function y = 1/x. The tangent to this curve drawn at the point
(1,1), will cut the x- axis at:
(A) (1,0) (B)(V2,0) (C)(1,V2) (D)(2,0)
9. A sample of water contains 200 ppm of Ca2+. What is the molality of the solution with respect to Ca2+? Atomic weight of Ca is 40.
(A) 0.2 m (B) 2 m (C)5xl0~3m (D) 0.05 m
10. The strongest Br0nsted acid among the following is
a OH
Pfvui COOH
11. Which of the following is necessary for a proccss to be spontaneous (AS = change in entropy)?
(A) ASxytnon 0 (B) ASsyttem 0
(C) AS universe > 0 (D) ASgumMindings < 0
12. If two vertices of a cube chosen randomly are painted black and the remaining are painted white, what is the probability that the black vertices are adjacent i.e. connected by an edge?
(A) 1 (B) | (C) (D)
2 7 7 28
13. C11I2 is unstable and it readily decomposes to
(A) Cu and F (B) Cu and I2 (C) Cul and I2 (D) Cul and T
|
cx (D) 'CH, |
15. The nickel-cadmium cell has a standard potential of + 1.20 V. The cell reaction is 2 NiO(OH) (s) + Cd (s) + 2H20 (/) -* 2 Ni(OH)2 (*) + Cd(OH)2 (i)
What is the standard free energy change for this reaction?
(A)-116kJ (B) - 38.7 kJ (C)-232kJ (D)-46.3kJ
s
16. If CosA = x; then CosAA -
(A)4x (B) 8x4-8 x2+l (C)4x4-4x2+1 (D)2x2+1
17. The packing efficiency in the hep, bcc and simple cubic (sc) lattices are in the order
(A) bcc > hep > sc (B) hep > bcc > sc
(C) hep > sc > bcc (D) sc > hep > bcc
|
18. The rate of decarboxylation of isomeric carboxylic acids is | |
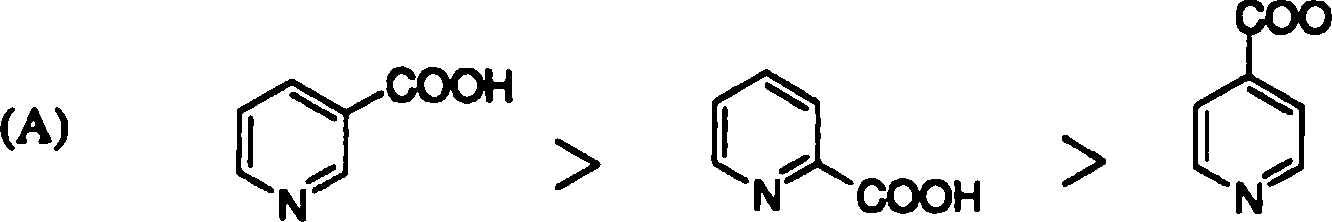 |
>H |
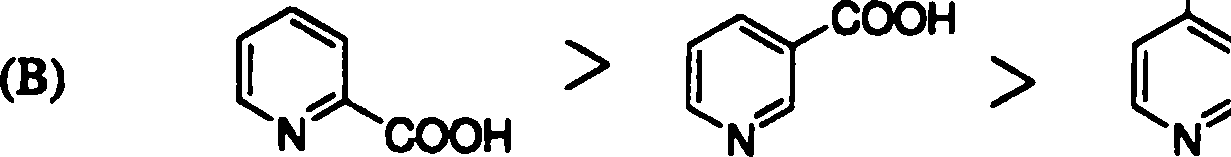 |
|
COOH |
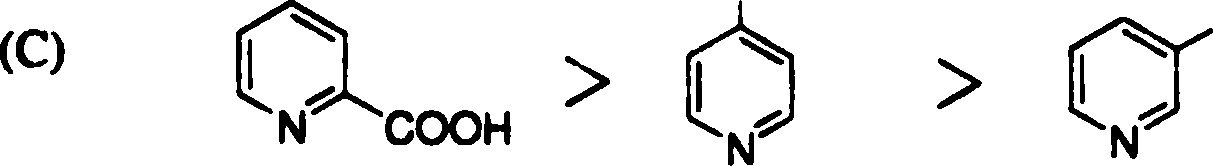 |
COOH |
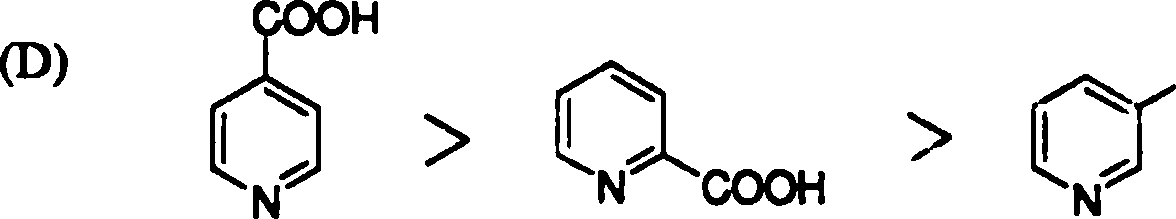 |
COOH |
19. Doubling all the coefficients in the equation for a cell reaction
(A) doubles E, but does not change AC0
(B) doubles AG, but does not change G
(C) does not change E or AG
(D) doubles both E and AG
20. The value of (l)y is
(C) e*
(B) i
(A) -1
21. The compounds ZnO and FeO show
(A) stoichiometric and metal excess defects, respectively.
(D) metal excess and stoichiometric defects, respectively.
22. An intermediate in racemization of (/?)-3-phenyl-2-butanone is
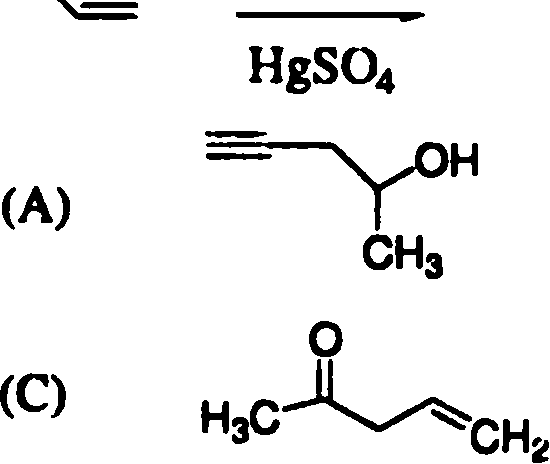
CH3
aq.EtOH O
aq. EtOH O
(*)
9*3 OH 0H
(A) Ph~9<. (B) PhV
H CH2 CH3
X*2 n HaC? ?H
(D) Ph-9f-OEt CH3 h ch3
Ha?
(C) Ph
23. Predict the sign of AS for both of the following processes
I. 2 C (graphite) + O2 (g) 2 C02 (g) n. C4H10 0?) C4H10 (/)
(A) AS should he negative for I and positive for II
(B) AS should be negative for I and negative for n
(C) AS should be positive tor I and positive for II
(D) AS should be positive for I and negative for II
jc4 + je3 + jc2 + X+1
24. The remainder of- is
jc-1
(A) 1 (B) 3 (C)5 (D) 7
25. Which two colors of light cause the highest rate of photosynthesis?
(A) Red and green (B) Blue and green
(C) Red and blue (D) Green and yellow
26. Assuming the additivity of covalcnt radii [C 0.77 A, Br 1.14 A], and assuming the distance between adjacent carbon atoms in the ring as 1.40 A, the distance between the ccntres of bromine atoms in 1,2-dibromobenzene is
(A) 3.31 A (B) 3.42 A (C) 4.20 A (D) 2.28 A
27. The number of stereoisomers for CHD=CH-CH=CHD is
(A) 4 (B) 8 (C)2 (D) 6
28. The entropy change associated with the expansion of one mole of an ideal gas from an initial volume of V to a final volume of 2.50 V at constant temperature is (R = gas constant)
(A) AS = - R In 2.50 (B) AS = - 2.50 R In (Vf/V*)
(C) AS = 2.50 R In (Vf/Vj) (D) AS = R In 2.50
29. The smallest among the following integrals is

I 1 !
(A) jexdx (B) je'r'dx (C) je ' dx
0 0 0
30. The quaternary structure of human hemoglobin is a
(A) dimer of two myoglobin dimers.
(B) tetramer of identical subunits.
(C) tetramer of four different subunits.
(D) tetramer of two different subunit*.
31. The number of isomers having non-zero dipole moment for PCI2F3 in the trigonal bipyramidal geometry is
(A) 2 (B) 3 (C) 1 (D) 0
32. The most appropriate reagent for the conversion of RCOOMe into RCH2OH is
(A) NaBFL* (B) LiBH* (C)NaH (D)Pd/CandH2
33. Which of the following statements must be true for the entropy of a pure solid to be zero?
(I) The temperature must be 0 K.
(II) The solid must be crystalline, not amorphous.
(III) The solid must be perfectly ordered.
(IV) The solid must be an element
(A) I, n and m (B) I and II (C) I (D) I, II, m and IV
34. The function with exactly two minima and one maximum, among the following is
(A) x4 - x2 - x (B) x + x2 - x4 (C) x3 - x2 - x (D)x + x2-x3
35. Collagen is
(A) an a-helical structural protein.
(B) a coiled-coil protein found in hair.
(C) a cross-linked globular protein.
(D) a triple-helical fibrous protein.
36. Given that 18F undergoes 90 % radioactive decay in 366 min., the half life (ti/2) for l8F is (A) 220 min. (B) 3473 min. (C) 154 min. (D) 110 min.
37. The phenolic compound among the following is:
(A) Ibubrufen (B) Paracetamol (C) Penicillin (D) Camphor
38. What is the hydroxide ion concentration of a solution that has a pH of 11.20?
(A)6.31xl0,2M (B) 11.20M (C) 1.58x1 O'3 M (D)2.80M
39. For all values of x which determinant among the following is zero?
|
X 1 |
(B) |
1 x |
1 X |
1 x2 | ||
|
1 X |
5 |
(C) |
X 1 |
(D) | ||
|
X JT |
X 1 |
40. The conductivity of sodium dodecyl sulfate (SDS) solution exhibits a sharp transition around 8 mM concentration. This is because:
(A) SDS precipitates beyond 8 mM concentration.
(B) SDS forms micelles above 8 mM concentration.
(C) SDS forms a gel above 8 mM concentration.
(D) SDS undergoes hydrolysis above 8 mM concentration.
41. According to the equation 2 Fe3+ + 21 - I2 + 2 Fe24
how many grams of iodine can be produced by reacting 7.4 mols of Fe3* and 7.0 mols of r ? [At. Wt. of iodine is 127]
(A) 8.9x10 (B) 9.1 x 102 g (C)9.4xl02g (D) 17.8x10
42. The most appropriate spectroscopy for the identification of a nitrile group is (A) IR (B)'HNMR (C)UV (D)ESR
43. If the units for rate are M s'1, what are the units for the rate constant, k, for a zeroth-order reaction?
(A) s'1 (B) M'V (C) M s'1 (D) M'1
44. The function with a finite range is
(A) e* (B) e** (C) ex' (D) e~*
45. How many grams of copper will be produced when 27 g of aluminium is added to excess cupric sulphate solution? [At. wts.; A1 = 27, Cu = 63.5]
(A) 63.50 (B) 90.50 (C) 95.25 (D) 122.25
46. The two strands of double helical DNA are associated by hydrogen bonds between adenine (A) and thymine (T), and between guanine (G) and cytosine (C). The numbers of hydrogen bonds between A-T and G-C pairs, respectively are:
(A) one, two (B) two, two (C) two, three (D) three, two
47. The intermediate involved in Curtius rearrangement is
(A) carbcnium ion (B) carbanion
(C) nitrene (D) carbene
48. An organic compound on decomposition at 500*C and 1 atm. pressure released 2 mL each of carbon monoxide, nitrogen and water vapour. The empirical formula of the molecule is
(A) CHNO (B) CH2NO (C) CH2N2O (D) CH2N2O2
49. -j-exp|/2] = at
(A) 1/r2 (B)exp(f2)
(C) 2/expC/2) (D) (mt)exp(t2)
50. Ncsslcr's reagent is prepared by mixing a solution of KI with a solution of X and then adding KOH solution. Here X is
(A) ZnCl2 (B) HgCl2 (C) AlClj (D) TiCl*
51. The intermediate acid involved in the following reaction is
A
NH4(CNO) - NHjjCONHj,
(A) uric acid (B) cyanuric acid (C) thiocyanic acid (D) cyanic acid
52. SO mL of 0.04 M HC1 solution was mixed with SO mL of 0.02 M AgNC>3 solution, stirred and filtered. The pH of the filtered solution is
(A) 1 (B) 2 (C) 3 (D) 4
53. (1 + 2i)'1 is equal to
(A) 1 - 2i (B) (1/5)-(2/5)/
(C)(l/3)-(2ri)i (D)-(1/3)+ (2/3)/
54. Which of the following fluorides is angular?
(A) BeF2 (B)ZnF2 (C)SnF2 (D)XeF2
55. The more reactive dienophile among the following for the reaction with cyclopentadiene is
.C02Et C02Et
( avrV
(A)
(C)
'V (D)
O
56. The magnetic quantum number of the last electron in the atom with atomic number 21 is (A) 4 (B) 3 (C)2 (D) 1
9i
57. If the number e is marked as a point on the complex plane, what is the distance of the point from the origin?
(A) 1 (B) 3 (C) 9 (D) tan19
58. What is the electronic configuration of V3+?
(A) [Ar]3d2 (B) [Kr]3d2 (C) [Ar]3d3 (D) [Kr] 3d3
59. The hybridization that is common for at least one of the carbon atoms in hydrogen cyanide, carbon disulfide, allene and carbon monoxide is
(A) sp (B) sp2 (C) sp3 (D) dsp:
60. Which one among the given functions has the smallest slope at x = 1?
(A) 2-3 (B)2-l (C)2x2-2jc (D)2x2-x
61. The order of basicity of the following substituted anilines is
NH2 NHo nh2
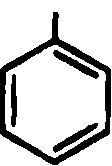

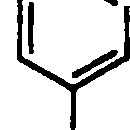
OjN NO2 N02
CH3 CH3
II III IV

(A)i>n>m>iv (B)iv>i>m>n (C)iv>n>i>in (D)rv>m>i>n
62. According to crystal field theory, the 9th electron of the metal centre in square planar [Cu(NH3)4]2+ resides in
(A) dxy (B)diS_y3 (C) dt, (D) dx
63. The rotation of pure /?(+)-Limonene is +123.0. Calculate the % of the (+) isomer in a sample showing a rotation of +109?
(A) 8.6 (B) 77.2 (C) 94.3 (D) 82.9
64. An ideal gas undergoes isothermal and reversible expansion from its initial volume to some final volume at 300 K drawing in 90 kJ of heat. The change in the Gibbs free energy of the gas is
(A) 300 J (B) ISO J (C)0J (D) -300 J
I
65.For0<x< 1, lim fnxedx = *
(A) 0 (B) 00 (C) (D) 2
66. An extensive property of a thermodynamic system among the following is
(A) Pressure (B) Temperature (C) Volume (D) Concentration
67. The product obtained in the following transformation is
(i) BH3-THF EtOzC C02H 00
H/Me H/, .Me
(A)
H02C C02H
lOaC CO?H
HMe H/Me

00 HCr "OH
68. The number of unpaired electrons in the complex ion is in the order
(A) CuCU2- < NiCU2- < ZnCU2' < CoCL,2"
(B) ZnCU2- < CuCU2- < C0CI42" < NiCU2"
(C) ZnCU2' < C0CI42' <NiCU2~< CuCU2'
(D) ZnCU2- < CuCU2- < NiCU2- < CoCU2'
69. If F(x) = xx then lim F(x) =
(A) 0 (B) 1 (C)qo (D) e
70. The metal ion involved in the water oxidation process at the active site of photosystcm
II is
(A) Mn (B) Mg (C) Mo (D) Fe
71. The oxidation number of carbon in dimethyl ether is
(A)-2 (B)-l (C) 1 (D) 2
72. The complex number -2 - 2V3i in polar form is given by
(A) 2e2*/3 (B) (C) Ae2 (D) 4e*%
73. Compound I gives a strong infrared absorption at 1730 cm*1. *H NMR spectrum indicates that it has two types of hydrogen atoms; one H atom appearing as singlet at 8 = 9.7 ppm and 9 H atoms appearing as a singlet at 5 = 1.2 ppm. The structure of I is
(A) HaCCHO (B) JtAcHO
H3C
CH3 rCH3
(C) H,CCH0 (D) JL
CH3 h3c cho
74. In a titration experiment, the end point indicates
(A) neutralization point (B) completion of precipitation
(C) apparent equivalence point (D) exact equivalence point
75. The stmctures I and II, shown below, correspond to:
COOH COOH
OH H
I II
(A) S-lactic acid and 5-alanine (B) /Mactic acid and /{-alanine
(C) /Mactic acid and 5-alanine (D) 5-lactic acid and /{-alanine
76. The heat of reaction of both the reactions 2 KOH + H2SO4 K2SO4 + 2HiO and Mg(OH)2 + H2SO4 MgS04 +2 H2O is -27.2 kcal/mol. Hence the heat of reaction of 3 Ca(OH)2 + 2 H3PO4 Ca3(P04)2 + 6 H2O would be
(A) -13.6 kcal/mol (B) -27.2 kcal/mol
(C) -81.6 kcal/mol (D) -68.0 kcal/mol
77. The standard equation of a circle passing through the points u (3,8), v (9,6) and w (13, -2) is
(A) (x - 3)2 + (y +2)2 = 100 (B) (x + 3)2 + (y +2)2 = 100
(C) (x - 2)2 + (y +3)2 = 100 (D) (x - 2)2 + (y - 3 )2 = 100
78. Acid is used in the standardization titration of KMnO* against sodium oxalate because
(A) it helps in dissolving KMn04.
(B) it stabilizes permanganate ion.
(C) it facilitates die reduction of Mn7+ to Mn2+.
(D)it helps in dissolving the Mn02 formed during titration.
79. Which of the following covalent compounds does not have any formally charged atom?
(A) (CH3)3NO (B) CH2N2 (C) CH3ONO (D) CH3CNO
80. The two radial nodes in the 3s radial function of H atom occur at the distances R1 and R2 from the nucleus. The three radial nodes in the 4s oibital occur at R3, R4 and R5. The order of these distances is given by
(A) R3<R1<R4<R2<R5 (B)R1 <R3<R4<R2<R5
(OR3<R1<R2<R4<R5 (D)R3 <R1 <R4<R5 <R2
81. The graph of the equation 4(x2 - 4x) 9 (y2 2y) - 29 = 0 represents a
(A) parabola (B) ellipse (C) circle (D) hyperbola
82. Using Wades rule predict the structure of B5H9
(A)closo (B)nido (C)arachno (D) scorpionato
83. In the SnI solvolysis of the following primary alkyl chlorides in aqueous ethanol, the order of decreasing reactivity is
II III IV
I
(A) i > u > m > iv (B) n > 1 > 111 > iv
(C)iv>m>n>i (D)m>n>i>iv
84. A solution of sulfuric acid contains 86 g of H2SO4 per liter of solution. The normality of the solution is
(A) 1.8 N (B) 0.9 N (C) 2.0 N (D)1.0N
85. The equation of the normal line to y = x3 - 2x2 + 4 at (2,4) is
(A) y = -~x+- (B) y = 9x + 4
4 2
(C) y = -4or+-| (D) y = -9x+-j
2 4
86. Which one of the following statements do not apply to interhalogen compounds?
(A) Could be neutral (B) Could be cationic
(C) Could be anionic (D) Always obey octet rule.
87. The product obtained by the reaction and two equivalents of Na is:
of one equivalent of 1 -bromo-3-chlorocyclobutane
(B) ci
(D)
(A) H (C) O
X
88. Which of the following pair has the lowest interfacial tension?
(A) n-decane/water (B) n-butan e/water
(C) air/water (D) n-octyl alcohol/water
89. The gas pressure in an aerosol container is 1.5 atm at 25*C. Assuming an ideal behavior of the gas, if the container is heated to 450C, the pressure would be close to
(A) 1.023 atm (B) 1.234 atm (C) 3.639 atm (D) 2.639 atm
90. The order of increasing dipole moment among H2S, H20 and BF3 is
(A) BF3 < H2S < H20 (B) H20 < H2S < BF3
(C) H2S < H20 < BF3 (D)BF3<H20<H2S
91. The best method for the following transformation is
(A) acid mediated hydration (B) hydrobcration-oxidation
(C) oxymercuration-demercuration (D) ozonolysis-reduction
92. The concentration of Ba2+ in saturated BaS04 solution at 27C is 1.04 x 10 * M. What is the solubility product (K*p) for BaS04 at this temperature?
(A) 1.04 x 1010 M (B) 1.08 x 10"10 M
(C) 0.52 x 1010 M (D) 2.08 x JO'5 M
93. What is the hybridization of sulfur in SF4?
(A) sp2 (B) sp3 (C) sp3d (D) sp2d2
94. The ester that undergoes acid hydrolysis most readily is
COjMs CO2M0
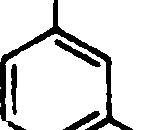
(A) f|l (B)
OMe 'Br
COjMe CXfeMe
95. If the half-life of a reaction is independent of its initial concentration, then the reaction may be categorized as
(A) zeroth order (B) first order
(C) second order (D) bimolecular
96. How many milliliters of 2 M NaCl solution are required to make one litre of 0.4 M NaCl solution by adding water?
(A) 5000 ml (B) 800 ml (C) 200 ml (D) 20 ml
97. Which of the following compounds is aromatic?
(A) Qbh (B) (Tbh
98. A 0.01 M solution of a compound transmits 20 % of visible light when the absorbing path length is 1.5 cm. What is the molar extinction co-efficient of the substance? Solvent is assumed to be completely transparent.
(A) 46.6 M'W (B) 50.3 M'em*1 (C) 22.3 M'W (D) 43.6 M'W
99. Which of the following atoms has the highest number of unpaired electrons in its ground state?
(A) C (B) N (C) O (D) F
100. Which of the following compounds has the highest boiling point?
(A) Mcsitylene (B) Benzene
(C) Toluene (D) Cyclohexane
18
|
Attachment: |
| Earning: Approval pending. |
