University of Hyderabad (UoH) 2011 M.Sc Bio Chemistry Entrance for (Biochemistry) - exam paper
M.Sc Entrance Examination, June 2011, Booklet Code: A
Time : 2 hrs Code No- V-12 ( M.Sc Biochemistry) Max Marks : 100
Please read'the following instructions carefully before answering
1. Enter the Hall Ticket number in the space provided above and also on OMR sheet.
2. Paper contains two sections: Part A and B together with 100 questions for 100 marks. Answer all questions and each question carries one mark.
3. Part A will be used for tie breaking.
4. There is negative marking. 0.33 mark will be deducted for each wrong answer. One mark is given to each of the appropriate or right answer
5. Answers have to be marked on the OMR sheet as per the instructions provided.
6. Apart from OMR sheet, the question paper contains 14 (Fourteen) pages including the instructions.
7. Please return both the question paper booklet and OMR answer sheet at the end of examination.
8. No additional sheets will be provided.
9. Rough work can be carried out in the question paper itself in the space provided at the end of the book let.
10. Non programmable calculators are allowed._
1. A positive A G (change in free energy) means that
A) reaction occurs spontaneously
B) the system is at equilibrium
C) no input energy required to drive such a reaction
D) Reaction cannot occur spontaneously and an input energy is required
2. Highest energy is associated with the following radiation
A) Gamma rays B) UV-rays C) Infrared D) Microwave.
3. In which of the following reactions new carbon-carbon bond is not formed:
B) Wurtz reaction
A) Cannizaro reaction C) Aldol condensation
D) Fridel-Craft reaction
4. A method that gives an idea of the three dimensional structure of low mol wt proteins
in solution
B) Nuclear Magnetic resonance
A) X-ray diffraction
C) Cryoelectron microscopy D) Circular dichroism
5. Which one of the following formulae does not represent an organic compound?
A) C4H10O4 B) C4He04 C) C4H7CI04 D) C4H904
6. Cananga odorata belongs to
A) Malvaceae B) Solanaceae C) Leguminosae D) Anonaceae
7. The second law of thermodynamics says that in acyclic process:
A) work cannot be converted into heat
B) heat can be converted into work
C) work cannot be completely converted into heat
D) heat cannot be completely converted into work
8. 2N HCI solution will have same molar concentration as a
A) 4.0 N H2S04 B) 0.5 N H2S04 C)1.0NH2S04 D) 2.0 N H2S04
9. Trehalose is a
A) Monosaccharide B) Disaccharide C)Trisachharide D) Polysachharide
10. Additiion of sodium acetate to 0.1 M acetic acid will cause A) increase in pH B) decrease in pH
C) no change in pH D) change in pH that cannot be predicted
11. A Lewis acid is a potential
A) electron pair acceptor B) electron pair donar
C) electron acceptor D) electron donar
12. Which one of the following will not give positive test for haloform reaction
A) Ethanol B) Propan-1-ol C) Propan-2-ol D) Butan-2-ol
13. Antigenic determinants of an antibody consist of:
A) variable regions of light chains only
B) variable regions of heavy chains only
C) variable regions of both heavy and light chains
D) constant regions of both heavy and light chains
14. Which of the following structures are superimposable
Me
BrJ-H HO~JEt Me
Me
Et
D) 1 and 3
H
BrIMe HOjEt Me 1
A) 1 and 2
Br
H-j-Me Et|OH Me 2
B) 2 and 3
H Me
OH 3
C) 1 and 4
Br
15. Beer -Lambert law relates absorption of light to any material through which a light source passes. Which one of the following is not a correct equation to express Beer-Lambert law
A) Log lo/l=e Cl B) A=eCI C) Log W =eCI D) Log T=eCI
Where, lo and I are intensity of light, T is transmittance of light, A is absorption of light, e is extinction coefficient, C is concentration and I is path length
16. Among the listed amino acids, which one is not an intrinsic fluorophore of protein/ peptide
A) Arginine B) Tyrosine C) Tryptophan D) Phenylalanine
17. Major interaction for protein structure stabilization is
A) Hydrophobic interaction B) Ionic interaction
C) Hydrogen bond interaction D) Van der-Waal interaction
18. Diameter of an coli bacterium can be approximately in the range of A) 0.5- 0.6 micrometers B) 2 - 5 micrometers C) 10-100 nanometers D) 1 - 5 nanometers
19. Light microscope resolves objects in diameter separated by approximately A) 300 nm and above B) 1-100 nanometers C) 0.5-1 nanometer D) 10- 50 nanometers
20. The cyanobacteria belong to which of the following kingdom?
A) Protista B) Plantae C) Fungi D) Eubacteria
21. Glycine is a A) ploar neutral amino acid C) polar negative amino acid
B) nonpolar neutral aminoacid D) non polar positive amino acid
22. Hydrolysis of ATP to AMP + PPi produces
A) less energy than intact ATP
B) more energy than hydrolysis of ATP to ADP and Pi
C) less energy than hydrolysis of ATP to ADP and Pi
D) None of the above are true
23. Arachidonic acid is
A) C18:1 B) C18:2 C)C18:3 D) C20:4
24. Cyanide inhibits electron transport at
A) Complex I B) Complex II C) Complex III D) Complex IV
25. In mammalian cells, processing of glycosylation occurs in the A) Endoplasmic reticulum B) Golgi apparatus C) Plasma membrane D) Lysosomes
QszrokLj- toJLi-' 4
26. Number of double bonds in palmitate A) Three B) Two C) Zero
D) four
27. Glucose 6-phosphatase enzyme activity is high in A) muscle cells B) Intestinal cells C) Liver cells
D) Heart cells
28. Which of the following is a non-protein amino acid A) Proline B) Alanine C) Ornithine
D) Glutamine
29. What is glutathione ?
A) alpha-glutamyl-cysteinly glycine B) gamma-glutamyl-cysteinly glycine
C) gamma-glutamyl-alanyl-glycine D) gamma-alanyl-glutamy-cysteine
30. Which of the following is an essential aminoacid ?
A) Tryptophan B) Tyrosine C) Alanine D) Glutamine
31. In Alkaptonuria the following enzyme is defective A) Homogenl.sic acid oxidase B) Homogentisic reductase C) Phenylalanine hydroxylase D) Adenyl Transferase
32. The basic structural unit of sphingolipids is A) Fatty acid B) Triglyceride C) Ceramide D) Sugarphosphate
33. Which of the following enzyme converts ribonucleotides to deoxyribonucleotides?
A) Ribonucleotide reductase B) deoxy Ribonucleotide reductase
C) thymidylate kinase D) HGPRT
34. Porphyrins are synthesized from
A) Alanine and succinyl CoA B) Glycine and succinyl CoA
C) Glutamine and acetyl CoA D) Glycine and acetate
35. Which of the following enzymes are used in the determination of primary sequence of proteins?
A) Trypsin and Chymotrypsin B) Enteropeptidase and hexokinase
C6(SIl-4
C) PNGase F 0) Catalase
36. In electrophoresis, proteins are separated based on
A) Charge and size B) Size alone C) Charge alone D) complexity
37. In prokaryotes, fatty acid breakdown occurs in
A) membranes B) cytosol C) peroxisomes D) mitochondria
38. Which of the following hormones play an important role in glucose metabolism?
A) Glucagon, Insulin, Epinephrine B) Prostaglandin,Norepinephrine.ACTH C) Insulin, Oxytocin,Thyroxine D) Thyroxine, Glucagon, Epinephrine
39. Which of the following does not contain SH group
A) Mercaptopyruvic acid B) Thiourea C) Homocysteine D) Cystathionine
40. Gout is a metabolic disorder related to
A) Nucleotide metabolism B) Protein metabolism
C) Lipid Metabolism D) Carbohydrate metabolism
41. The target for aspirin is
A) Acetyl CoA carboxylase B) Cyclooxygenase
C) monooxygenase D) mixed function oxidase
42. Identify the correct statement
A) The limiting amino acid in maize is Lysine alone
B) The limiting amino acids in maize are Lysine and threonine
C) The limiting amino acids in maize are tryptophane and threonine
D) The limiting amino acids in maize are Lysine and Tryptophan
43. Which one of the following vitamins participates in citric acid cycle A) Bi B) Be C) Bs D) Folic acid
44. Incompatible blood transfusion leads to
A) Hemolytic jaundice B) Obstructive jaundice
C) Hepatic jaundice D) Regurgitation jaundice
|
45. Wilson's disease is due to A) Toxicity of copper C) Deficiency of iron |
B) Deficiency of copper D) Toxicity of zinc |
46. Epigynous flowers are found in A) Pumpkin B) Datura
C) Magnolia
D) Rose
47. Plants having separate male and female plants are caiied
A) Monoecious B) Monogamous C) Polygamous D) Dioecious
48. Entire leaf is modified into tendril in
A) Lathyrus B) Gloriosa C) Nepenthes
D) Smilax
49. When a nonvolatile solid is dissolved in a liquid, the vapour pressure and freezing point of the liquid:
A) Both decrease B) increases and decreases respectively
C) Both increase D) Decreases and increases respectively
50. In plants, when the ovule is erect so that funicle, chalaza and micropyle lie on tiie same vertical line called
A) Orthotropous ovule B) Amphitropous ovule
C) Circinotropous ovule D) Campylotropous ovule
51. The stamens in which the anthers or the filaments are not fused, they are called
A) monadelphous B) syngenesious C) polyadelphous D) polyandrous
52. Alu DNA sequences originated from A.) Ribosomal RNA B) 7SL RNA,
D)U5RNA
C) U1RNA
53. Which of the following set of quantum numbers (n, 1, m and ms) is valid one for hydrogen atom ?
A) 3. 0,-1,-1/2 B) 2, 3,0,+1/2 C) 5, 2,1,+1/2 D) 3, 2, 3,+3/4
54. L-shaped tRNA represents
A) primary sequence B) secondary structure
C) tertiary structure D) quartenary structure
55. The fruit of mango called
A) Pome B) Drupe C) Hesperidium D) Berry
56. Gaseous H2 and l2are reacted to give gaseous HI. On which of the following does equilibrium constant Kp depend?
A) Initial pressure of 12 B) Temperature
C) Total volume of the reaction vessel D) Total pressure of the system
57. In biological protein synthesis, transfer RNA plays a role
A) as a template
B) recognizes 5 end of messenger RNA
C) as a constituent of ribosome that offers a surface for carrying out protein synthesis
D) recognizes a codon in the template
58. E.coli is a
A) grampositive, anaerobic organism
B) gramnegative, aerobic organism
C) grampositive, anaerobic and sporulating
D) gramnegative, facultative anaerobic and non-sporulating
59. RNA polymerase plays a role in
A) DNA synthesis using RNA as template
B) RNA synthesis using DNA as a template
C) RNA synthesis using RNA as a template
D) Synthesis of protein using RNA as template
60. Calcium, in cells, is stored in
A) mitochondria B) nucleus C) golgi D) endoplasmic reticulum
61. An endonuclease cuts
A) RNA B) DNA C) RNA or DNA internally D) RNA or DNA from the ends
62. In volvate aestivation
A) sepals touch with overlapping
B) sepals touch without overlapping
C) sepals overlap but do not touch
D) sepals do not touch or overlap each other
63. Cone cells are important in sensing
A) smell B) light C) colour D) Taste
64. Somatostatins are
A) small chemicals produced by neurons that inhibit nerve impulses
B) small peptide hormones that inhibit growth
C) growth stimulating hormone
D) None of the above are true
65. The specificity of bacterial RNA polymerases for their promoters is due to which subunit?
D) a
C) y
A) a
66. Which of the following sequence modules is NOT a basal promoter element?
A) CAAT box B) GC box C) Octamer module D) TATA box
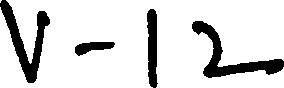
67. The chemical modification of eukaryotic rDNA molecules takes place in
A) cytoplasm B) endoplasmic reticulum C) nuclear envelop D) nucleolus
68. Which of the following statements about telomerase is true?
A) Telomerase is an RNA-dependent DNA polymerase
B) Telomerase is an RNA-dependent RNA polymerase
C) Telomerase is an DNA-dependent DNA polymerase
D) Telomerase is an RNA-dependent RNA polymerase
69. What protein is involved in the separation of the two interlinked daughter chromosomes when DNA replication is terminated in E. coll?
A)DNAB B) DNA polymerase C) Topoisomerase IV D)Tus
70. In the N-terminal regions of histone protein, which aminoacid is acetylated
A) Arginine B) Lysine C) Serine D) Tyrosine
71. Which is NOT TRUE about the lac repressor
A) It is induced by exposure of a bacterial cell to lactose
B) It uses the same promoter as the lacZ gene
C) It changes the shape in the presence of inducer
D) It can form alternate stem-loop structures
72. A bacterium containing sodium ions at a concentration of 0.1 mM lives in a pond that contains sodium ions at 0.005 mM. Evidently, sodium ions are entering the cell by
A) active transport B) endocytosis C) diffusion D) osmosis.
73. The extreme 5 end of a prokaryotic mRNA has a
A) Shine Daigamo sequence B) methylated guanosine
C) phosphate D) hydroxyl group
74. Cytochrome P450s come under which of the following sub-class of oxidoreductases
A) Oxidases B) Oxygenases C) Peroxidases D) Dehydrogenases
75. Molecular action within biological membrane is best characterized by which of the following statements?
A) Lipid molecules readily flip-flop" from one side of the membrane to the other
B) Lipid molecules exhibit lateral movement within the membrane bilayer
C) Protein molecules are all situated on the cytoplasmic surface of the membrane bilayer
D) Lipid molecules do not exhibit any kind of movement within the membrane bilayer
76. Which of the following membranes would be the most fluid?
A) a bilayer made of lipids with polyunsaturated 18 carbon-fatty acids
B) a bilayer made of lipids with saturated 18 carbon-fatty acids
C) a bilayer made of lipids with saturated 16 carbon-fatty acids
D) a bilayer made of lipids with polyunsaturated 16 carbon-fatty acids
77. Of the following membrane lipids, which is not found in prokaryotes?
A) Phospholipids B) Glycolipids C) Cholesterol D) Diacylglycerol phosphate
78. What is the pH optima of pepsin
A) 2.0 B) 4.0 C) 7.0 D) 8.0
79. Which of the following statements is incorrect to a cancer cell
A) increased contact inhibition B) increase transport of glucose
C) ability to proliferate indefinitely D) high saturation density
80. The following character is exclusively found in mammals
A) Homeothermy B) Internal fertilization
C) Muscular diaphragm D) Four-chambered heart
81. Atmospheric nitrogen is fixed non-symbiotically by which of the following genera?
A) Nitrobacter B) Azotobacter C) Nitrosomonas D)Rhizobium
82. Birds have bipedal locomotion because
A) Reduces the weight B) Frees forelimbs for flight
C) Supports the body better D) Quickens the pace
83. Which class of enzymes catalyze the following reaction: A + B = A-B where the covalent bond formed between A and B is coupled to ATP hydrolysis
A) Isomerase B) Lyase C) Ligase D) Transferase
84. The genotypic ratio of F2 progeny of a monohybrid cross with alleles Aa will be A) 3:1 B) 1:2:1 C)2:1 D)4:0
85. The following statement is not-true
A) the conjunctival epithelium is derived from the mesoderm
B) the maxillary process forms the lower eyelid
C) the Schlemm canal is present in the first trimester
D) the drainage of aqueous through the trabecular meshwork begins at gestation
86. In anaphase I
A) sister chromatids move to opposite poles
B) the chromosomes align at random at the centre
C) the homologues move to opposite poles
D) crossing over of homologues takes place
87. Haploids are useful in genetic analysis because
A) their division cycle is faster due to reduced chromosome number
B) they are easy to maintain
C) all mutations are easily detected
D) tetrad analysis can be done
88. The maternal parent of an organism contributes
A) only mitochondrial DNA B) only chloroplast DNA
C) only nuclear DNA D) all of the above
89. Riboflavin is
A) Vitamin B1 B) Vitamin B2 C) Vitamin B5 D) Vitamin B12
90. The chromosome complement of a germ cell is
A) same as other body cells B) half of other body cells
C) twice as of other cells D) intermediate between half and full complement
of other cells
91. The disease, Atheletes foot, is caused by
A) Virus B) Bacteria C) Fungus D) Mycoplasma
92. Colour blind males are more common than colour blind females. The most likely reason for this is
A) the gene for colour blindness is carried on the Y chromosome
B) females are resistant to color blindness
C) the gene for colour blindness is carried on the X chromosome 0) male specific hormones cause colour blindness
93. Acetyl Co-A is produced in
A) mitochondrial matrix B) cytosol
C) in the lumen of endoplasmic reticulum D) golgi
94. Jellyfish, hydras, sea anemones, Portuguese man-of-wars, and corals belong to which of the following phylum?
A) Porifera B) Mollusca C) Echinodermata D) Cnidaria
95. Oxyntic cells are located in
A) islets of Langerhans and secrete glucagons
B) gastric epithelium and secrete pepsin
C) gastric epithelium and secrete HCI
D) kidneys and secrete rennin
96. Chemical DNA sequencing method was developed by
A) PSI Nyr6n and Mostafa Ronaghi, B) Sanger
C) Walter Gilbert and Allan Maxam D) James watson
97.100 Angstroms mean
A) I nanometer B) 5 nanometers C) 10 nanometers D) 100 nanometers
98. The bacteria that cause Diphtheria in man belong to
A) Mycobacterium B) Yersinia C) Corynebacterium D) Klebsiella
99. Cleavage of an IgG molecule by the protease, papain, produces:
A) an antigen-binding site and two constant regions
B) two heavy chain-light chain dimers.
C) an inactive mixture of oligopeptides.
D) two Fab fragments and one Fc fragment
100. Which of the following is NOT a specific immune response?
A) T cell proliferation B) antibody mediated complement activation
C) antibody production by plasma cells D) acute inflammation
14
|
Attachment: |
| Earning: Approval pending. |
