Biju Patnaik University of Technology 2009-2nd Sem B.Tech (B Tech) ,Chemistry - Question Paper
2009 Biju Patnaik University of Technology B.Tech BPUT(B Tech) , second semester Chemistry ques. paper
immersed in solutions of Zn*s ions of 0.1 M and 0.01 M concentrations. 3
7. (a) Show that the radius ratio value for
co-ordination number 3 of a solid is 0.1.
(b) Define Law of Rational intercepts for a crystal. 3
\c) 2 moles of HI were heated in a sealed tube at 440 K till the equilibrium state was reached. HI was found to be 22% dissociated. Calculate the equilibrium constant for the dissociation reaction and also calculate the standard free energy change for the reaction.
8. (a) Define Hess's law of Heat of Summation.
(b) Write down the EMF expression by representing the cell for a reversible cell in which the following reaction persists.
AgCl(s) +1 /2H2(g) Ag(s) + HCI(aq)
3
(c) Determine the Miller indices for the plane with its intercepts along the axes are 2a, 3b and 2c respectively. 4
o
(c) NO is paramagnetic and NO+ is diamagnetic. Using MO configuration justify.
(d) The metal ion deficient defect MX1+& is not possible. Justify.
(e) The rate law for the decomposition of N205 is given by {Rate = k[N205]}. Where, k = 6.2 x lOsec-1. Calculate the half-life and average life of the reaction.
(f) Calculate the equilibrium Keq for the following reaction at 27QC :
2Ag + Zn Zn+2 + 2Ag
Where the standard emf of the cell is 1.56 V.
(g) What is the physical significance of free energy change (AG) ?
(h) Write down vn't Hoff reaction isotherm giving the significance of all the terms
involved there in.
What role does As play in the synthesis H2S04 by contact process.
When NH4CI is heated alone in a closed vessel it is 1 component system, where as when heated either in presence of NH3 or HCI then it is two component system. Justify your answer
(i)
(a)
(b) (c)
Show that the variation of free energy of a system with temperature is a measure of decrease in the entropy of the system at constant pressure. 4
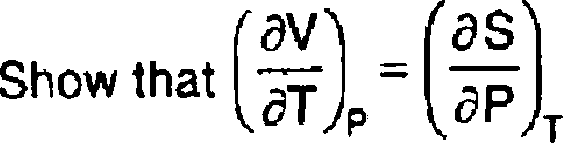
Calculate the change in entropy (AS) when 1 mole of water is vapourized at 100 C and 1 atmospheric pressure. The latent heat of vaporization of water is 540 cal /gm. 3
|
+ P | ||
|
Uvj |
T |
,3T, |
(b) Derive Gibb's-Helmholtz equation 5
3. (a) Show that CP - Cv =
4. (a) Define phases, components and Degrees
of Freedom. Give appropriate examples to support the definition. 6
(b) Disacuss the phase diagram of Cd-Bi system. 4
5. (a) The half-life of a reaction become 2.5
times if the concentration is made half. What is the order of the reaction ? 3
(b) At 300 K, a 1sl order reaction is 50% completed in 20 minutes. At 350 K, the same reaction is 50% in 5 minutes. Calculate the energy of activation of the reaction. 3
(c) The plot of l/(a-x) versus time for a particular reaction is given as follows :
Derive the expression for the rate constant of the reaction. 4
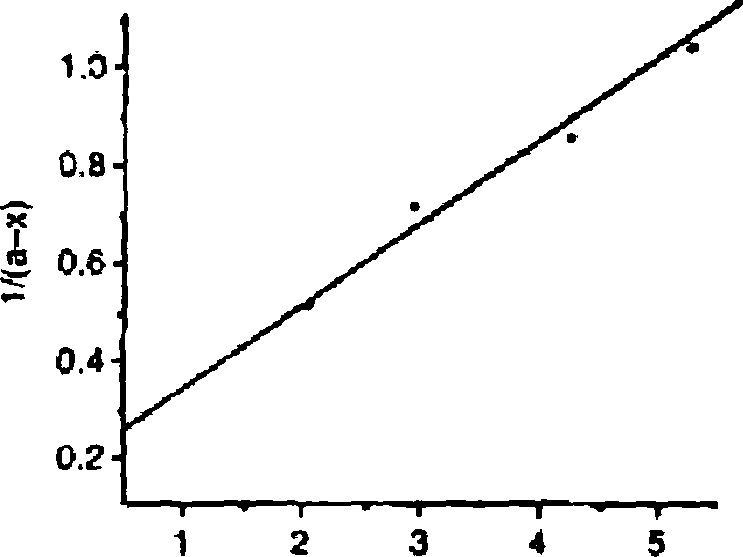
tuorr
Time
a
m
c
Discuss the work/ng of a calculator battery giving the cell reaction involved there in,
4
(b) What are the main advantages of alkaline battery over dry battery ? 3
(c) Calculate the EMF of a concentration cell at 25C consisting of two Zn electrodes
V
jbput
u
Total number of printed pages -7 B. Tech
BSCC 2101/BS 1103
Second Semester Examination - 2009 CHEMISTRY - I
. Fgll Marks -70
Time: 3 Hours
Answer Question No. 1 which i$ compulsory and any five from the rest.
The figures in the right-hand margin indicate marks.
1. Answer the following questions : 2x10
(a) State Heisenbergs uncertainty principle.
(b) Calculate the kinetic energy of an electron emitted from a surface of a metal, by the irradation of light of frequency 5.5 x 1017sec1. The work function of the said metal is 3.62 x10-12 erg.
|
Attachment: |
| Earning: Approval pending. |
