University of Delhi 2010-2nd Year M.Tech Information Technology 1st nd sem chemical synthesis and process technologies - Question Paper
This question paper contains 6 printed pages.
r
7646 Your Roil Mo..................
CHEMICAL SYNTHESIS AND PROCESS TECHNOLOGIES
Paper - 202
(Solution Chemistry & Catalysis in Chemical Synthesis)
Time 3 hows Maximum Marks 70
(Write your Roll No on the top immediately on receipt of this question paper J
Use separate answer scripts for Section A & Section B. SECTION A
Attempt three questions in all, including Q No 1 which is a compulsory question Q No 1 carry 11 marks and rest of the questions carry 12 marks each Use of Scientific Calculator is allowed
1 a) Define (j), a, & iTfor the following reaction Cu2+ + 4NH3 [(Cu (NH3)4]2+ which takes place in various steps Derive a relation between these functions. * 03
b) (i) Define masking
00 Why conditional stability constants are more important than stoichiometric stability constants in complexometric titrations 03
c) Small amounts of Magnesium are to be determined complexometncally in the presence of large amounts of
Zinc The titration is performed with EDTA at pH = 10 with Eriochrome black T as indicator after the Zinc has been masked by means of tetren (tetraethylene pentamm = T) added in approx 0 1M excess Estimate the accuracy of visual titration in
(a) 1 O'2 M Mg in the presence of 0 1M Zn
(b) 1 O'3 M Mg in the presence of 0 1 MZn Given log ay(H) = 0 5, log aZn(T) =14 0
loSKZy=165> l08KMgy=87-
PM8.ras = 5 4 05
2 a) What do you understand by back titrations When it is necessary. 04
Show that
[ML] [M] K [M]
TE = 11
[M,L] [M] kMi|L [ML] kM[L [M,L]
b) What is the most suitable conc.of added Mg y when titrating calcium in 1 O'3 M conc with EDTA at pH=l 0 using EBT as an indicator Given that
pM, -5 4, pCa - 6 6 04
r trans r eq
c) What do you understand by metal lochromic indicators 7 Prove that
a) Calculate the conditional stability constant of calcium complex with EDTA at pH= 10 Given that log KCa EDTA =10 7 The protonation constants of EDTA are
log KH) = 10 34, log KH) = 6 24,
log K3(H) - 2 75, log K4(H) - 2 07 04
b) A metal ion Mn+ has been estimated spectrophotometrically using legend L according to the following reaction
mM + nL MmLn
Using Jobs method of continuous variation, show that, in a plot of absorbance (A) of the complex Vs mole fraction of legend (x), the maximum into the plot
x n
corresponds to=
1 -x m 04
max
c) The formation of the 1,10 - phenanthroline complex of cadmium is investigated by the polasographic method. The total cone, of cadmium and the concentration of supporting electrolyte, KN03, are constant, whereas the concentration of 1, 10 - phenahthroline is varied. All the solution contain 40% ethanol and the pH is 6 6.
cal \ KNOj
Protonation constant of 1,10 - phenanthroline
log k = 4 95 \
The half wave potentials measured against SCE and determined from the polarograms, the wave heights in mm, are as follows.
|
T(phen)M |
E'/aOO |
id(mm) |
|
0 |
-0.591 |
170 |
|
10'3 |
-0 745 |
135 |
|
2x 10'3 |
-0 790 |
122 |
|
4 x 10*3 |
-0 822 |
120 |
|
10 x 103 |
-0 862 |
113 |
|
20 x 10*3 |
-0 895 |
113 |
Determine the composition & stability constant of the complex formed 04
4 a) The complexation reaction
M + nHL->ML + nH+
which occurstn stepwise manner has been investigated by pH Metric method If TL & TM are total legand conc & total metal conc resp , show that [H+]
pL = log[li5i_
T
M
04
b) In the solvent extraction technique for the determination of the stability constants of the complex Show that log g = log ac + log Pc (3n++ (C -N) log L in the presence of large excess of legand, where g the distribution ratio of metal in two phases, pN & (3c are overall formation constants for MLV. & ML
N C
complexes respectively
Calculate the % of metal (M) present as free ion and as thiocyanato and hydroxo complexes in solution which is 0 1M potassium thiocyanate and contains a small concentration of M, at pH 7 as well as at pH 9 The overall stabihn constants are as follows -For Thiocyanato complexes,
log P = 1 2, log P2= 1 6 & log P3= 1 8 For Hydro complexes
Attempt all questions
1 Explain the following by taking suitable examples 3x3 = 9 x
Generation of coordinatively unsaturated metal centre is
an important step in catalysis
(n) a- and 7t-bonding is essential for transition metal ions to act as good catalyst
(iii) Tolman parameters to measure the electronic and stenc effects.
2 Attempt any two of the following* 2 x 5 - 10
(i) Suggest a catalyst and explain the catalytic cycle involved in the conversion of allyl alcohol into propionaldehyde
(ii) Discuss the mechanism of catalytic conversion of ethylene to acetaldehyde using PdCl2 as a catalyst
(iii) Discuss the mechanism of heterogeneously catalyzed Fischer- Tropsch reaction
3. Explain any two of the following: 2x5 = 10
(i) Explain hydroformylation of alkene with mechanism and suitable example
(n) What do you understand by the term stereo regular polymerization? Discuss the catalytic cycle of Zeigler-Natta stereo regular polymerization of propylene.
(ni) Explain the mechanism of hydrocyanation reaction of propene using Ni-catalyst.
Pd(OAc)
BuOCH = CH, + PhBr --> 2 NEt3
Ionic Liquid
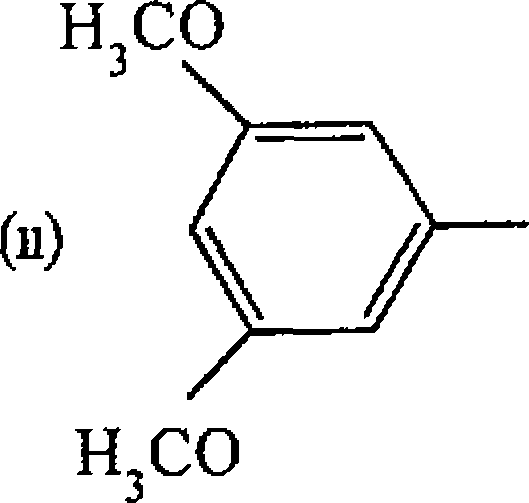
Pd(OAc)2
Br + CH, = CH, P(-tolyl)3_*
2 2 ' NEt,
pdCl2 CuCL fin) CH,CH,CH = CH -2
3 2 2 h2o/ o2
Pd2+ (iv) CH3C = CH + CO + CH3OH 2.pyr,dylphosph,-ne>
Pd (II) complex
(v) CH2 = CHCH2Br + CO + CH3CH2OH ->
Ni- cat
(vi)
HCN, hexane
|
Attachment: |
| Earning: Approval pending. |
