University of Delhi 2010-2nd Year M.Tech Information Technology 1st nd sem reagents in organic synthesis newer synthetic reactions and methodologies UNIVERSITY - Question Paper
[This question paper contains 6 printed pages]
Your Roll No
7645 J
M.Tech./II Sem.
CHEMICAL SYNTHESIS AND PROCESS t TECHNOLOGIES
Paper-201-Reagents m Organic Synthesis, Newer Synthetic Reactions and Methodologies
Time 3 Hours ' Maximum Marks . 70
(Write your Roll No on the top immediately on receipt of this question paper )
Use separate answer script for section A and B SECTION-A
Answer all questions, Do not discuss mechanisms unless asked for State the principle and/or concept involved m the reactions Write neat perspective structural diagrams 1 (a) How is Wilkinson's catalyst prepared9
(b) When an alkene (RCH=CHR) is hydrogenated using a mixture of D2 and H2, the product (RCH2CH2R) contains molecules RCHD CHD R and RCH2CH2R only9 There are no molecules containing D and H (RCHD CH2R) Account
How is borane prepared from sodium borohydride9 Compare alkyl boranes and Gngnard reagents in their chemical reactivity
Select a hydride transfering agent which can be used to convert a carbonyl to methylene Write all the steps
(c)
(d)
2 (a)
(b)
(c)
Compare the reactivity of sodium borohydnde and LAH towards a conjugated ketone Solve any two
|
(i) | 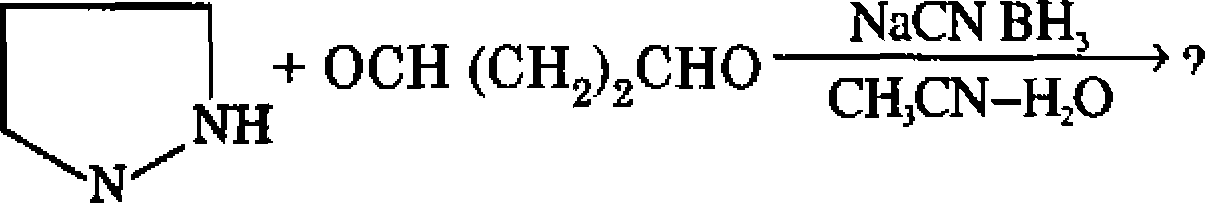 |
|
H | |
 |
|
D-limonene |
THxBH.
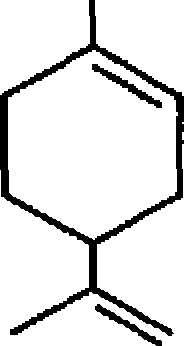
(u)
|
CH3 | ||
|
H,0,-Na0H | 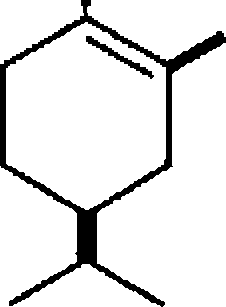 |
OH (-) Carvomenthol |
CHjCOOH
1 mole A
PhCOCRBr ) ? BOH + Bu'OK
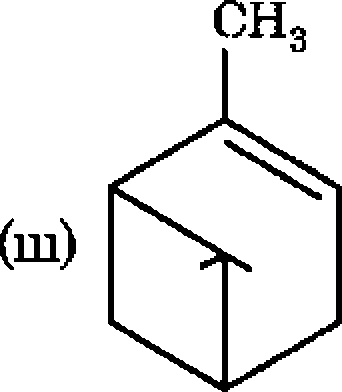
(a) Solve the following problems based on the concepts of vinyl silanes and allyl silanes
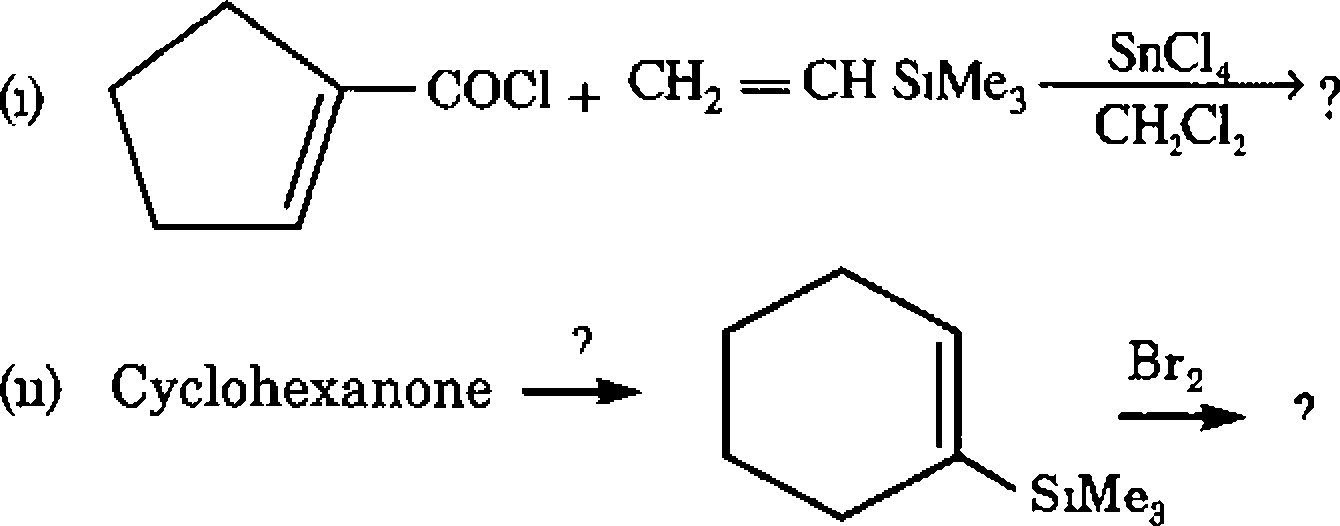
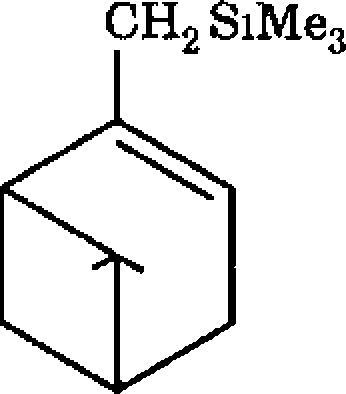 |
CHgCOCl -> AlClg 90 C |
(b) Recall the chemistry of enol silyl ethers, work out the following
/XOSiMeg
(1) Cyclohexanone
CH2=NMe* f
(I) Peterson Reaction
(II) Wacker Oxidation (m) Heck Reaction
(iv) Suzuki Cross coupling
(v) Trialkyl silyl halides (Cl, Br, I) in organic synthesis
SECTION-B Attempt any five questions Explain the term Umpolung synthesis Give the synthesis of 4-methylpent-3-ene involving acyl anion equivalent of
1,3-dithiane 2+5
Write the mechanism of any two of the following reactions 3 5+3 5
(I) Arbuzov rearrangement
(II) Mukaiyama-Johnson aldol reaction
(III) Horner-Wadsworth-Emmons reaction or Wittig reaction of stabilized yields
( 5 ) 7645
Predict the products (s) formed in each of the following reactions 2+5
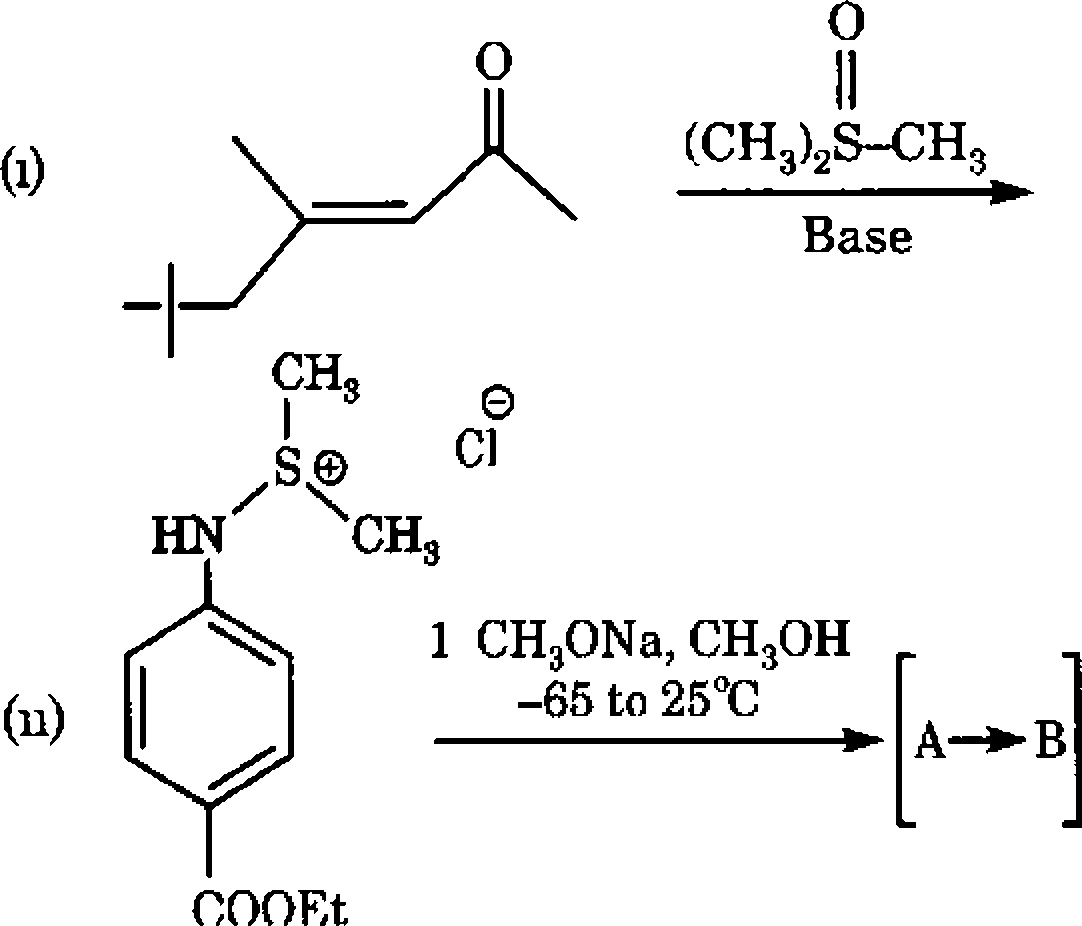
raney Ni C- D
Explain any two 3 5 + 3.5
(l) Why enamines are nucleophilic7 Give one example (n) Why clay-supported PTC is better than polymer-supported PTC7 (m) Why Z-enolates give syn-aldol products and E-enolates give anti-aldol products7 Explain on the basis of Zimmerman-Traxler model Explain the action of the mimic of Ribonuclease A system in the hydrolysis of the phosphates 7
|
O |
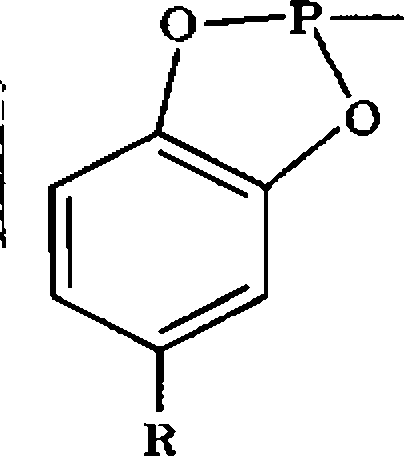 |
|
R = CH, or C(CHq), |
6 Predict the product formed by the reaction of acrylamide (a weak Michael acceptor) and aromatic aldehydes such as 2-formylthiazole at room temperature in an aqueous medium (l,4-dioxanel/H20) (1 1) in the presence of a stoichiometric amount of DABCO Write the name of reaction involved and its mechanism 3+1+3
7 (a) The sulphomum salt [B] formation was carried out
by treating the precursor thiol [A] with C7H7Br [C], followed by addition of AgC104 The reaction of B with D (aldehyde) gives the compound E and recovered compound A Complete the reactions involved m this synthesis
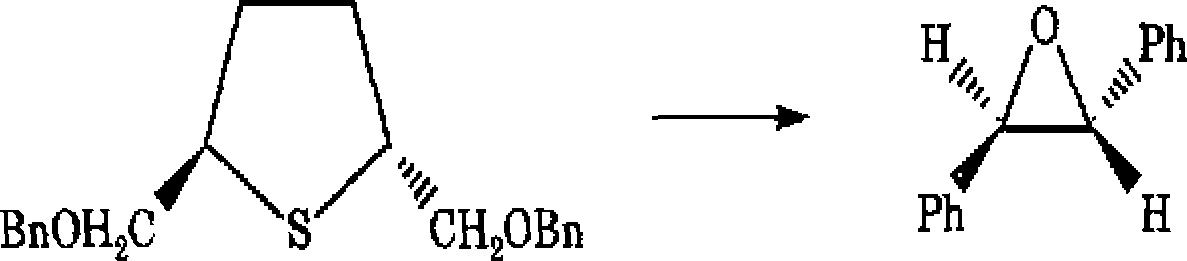 |
+ A |
|
A E | |
(b) Write structures for the following compounds
(i) DBU
(n) Common ammonium or phosphomum salts used
as PTC
(m) Dicyclohexano-18*crown-6
4+3
100
|
Attachment: |
| Earning: Approval pending. |
