University of Delhi 2010-2nd Year M.Tech Information Technology 1st nd semspectroscopy II and heterocyche chemistry UNIVERSITY - Question Paper
[This question paper contains 9 printed pages ]
Your Roll No
M.Tech./II Sem.
CHEMICAL SYNTHESIS AND PROCESS TECHNOLOGIES Paper-203 Spectroscopy-II and Heterocyclic Chemistry (Chemical Synthesis and Process Tech)
Time 3 Hours Maximum Marks 70
{Write your Roll No. on the top immediately on receipt of this question paper )
Use separate answer sheet for section A and B.
SECTION-A Attempt all the questions
1 Attempt any six of the following (answer should not be more than 2-3 lines) 2x6
(a) Ethyl benzene gives base peak at m/z 91, while propyl benzene gives two peaks at m/z 91 and m/z 92 Explain
(b) An unknown compound has a molecular ion peak at m/z 110 with relative intensity of 100% The relative intensity of M+l peak is 6 5% and that of
7647 ( 2 )
M+2 is about 4 7% Calculate molecular formula of the compound
(c) Methyl carbon of acetonitrile appears at 1 79 ppm m 13 C NMR, while methyl carbon of methyl chlonde appears at 28 7 ppm, even though cyno group has higher electronegativity than cyno group (dipole moment of acetonitrile is 3 92D, while methyl chlonde has dipole moment of 1 85D) Explain
(d) A compound shows M+ peak at 186 (100%), M+2 peak at 188 (195%), and M+4 peak at 190 (95%) It shows only one signal in the 1H NMR Identify the compound
(e) How will you distinguish between isomeric alcohols of molecular formula C5H120 by MS
(f) How will you differentiate between propyne and but-2-yne by 13C NMR
(g) How will you differentiate between o - to iyl-methanol and p-tolyl-methanol by MS
(h) How many signals do you expect for 2,2,4-trimethyl-
1,3-pentanediol in 13C NMR
An aromatic hydrocarbon shows molecular ion peak at m/z 121 Find out molecular formula of the compound
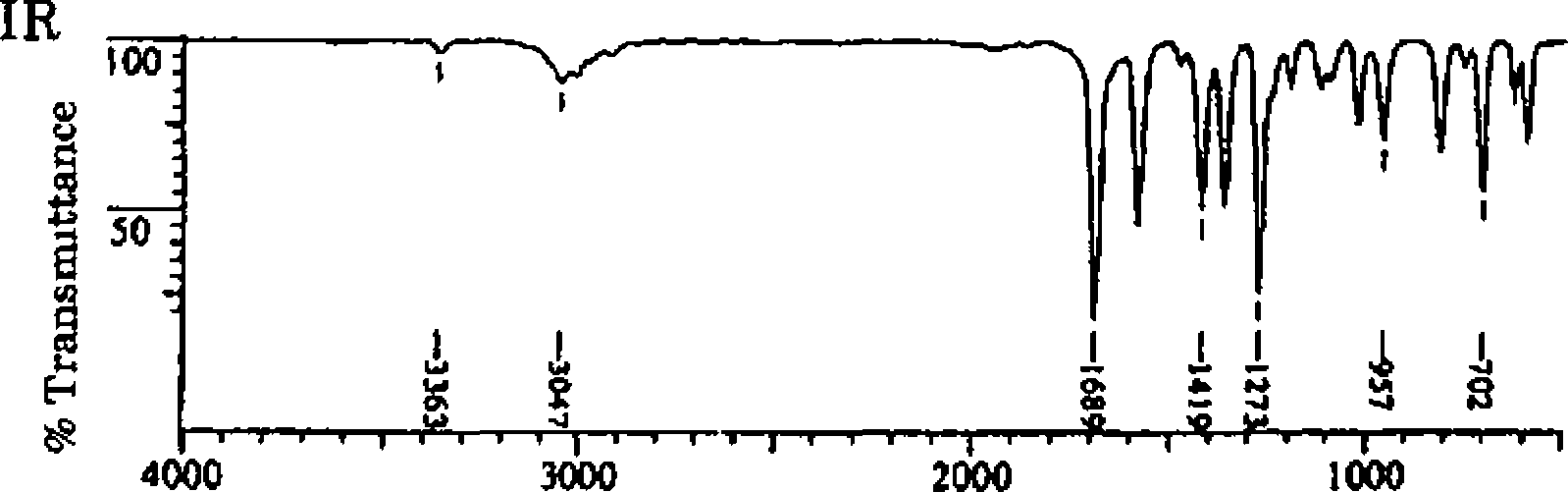
2 IR, NMK, 13C NMR and mass spectral of an unknown compound are given in chart A Deduce the structure of the compound, assign the peaks, and show the mass
spectral fragmentation
12
Or
(a) A compound of MF C13H20O2N2 gave following spectral data IR 3500, 3400, 1735 (cm"1), NMR
1 15 (t, 6H), 2 4-2 8-1 9 (m, 6H), 3 70 (brs, 2H),
4 10 (t, 2H), 6 8 (d, J = 8Hz, 2H), 7 8 (d, J = 8Hz, 2H),1 13C NMR 13 7 (+), 46 4 (-), 53 2 (-), 66 2 (-), 115 (+), 120 5 (Cquart), 130 4 (+), 151 2 (Cquart), 167 2 (Cquart), MS (m/z) 236 (M+), 235, 207, 164, 150, 121 Find out structure of the compound and assign all the peaks 8
(b) A compound of MF C7H?NO gave following spectral data lK NMR 2 83 (m, 2H), 3 82 (t, 1H), 7 52 (d, J = 8Hz, 2H), 8 66 (d, J = 8Hz, 2H), 13C NMR
48 8 (-), 57 3 (+), 123 2 (+), 149 7 (+), 152 7 (Cquart), MS (m/z) 121 (M+) Find out structure of the
compound
4
ll*-]
( 4 ) CHART A
ioo
n 50
W
O
$
|
1111 i > [ f'H 11 111 11 ttIt] riii|i[ii[iiM]iiii|Mii|ini|ii rrprn i [ H 11 [ rn i*| i 111 50 60 70 80 , 90 100 110 120 m/z |
 |
|
Wavenumber (cur4) |
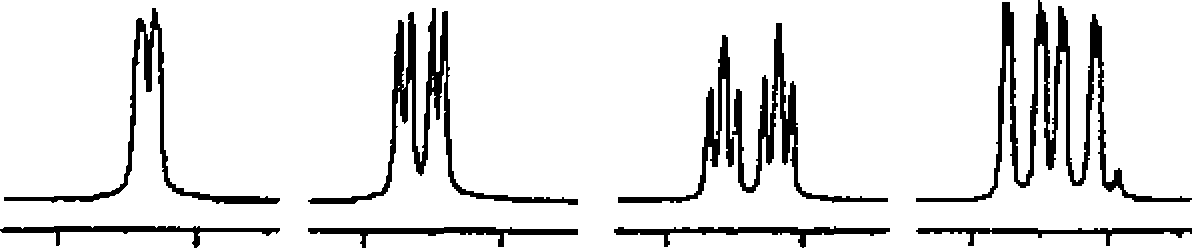
!H NMR 300 MHz
 |
|
2720 2700 Hi 2600 2380 Hi 2440 2420 Hi 2200 21 SO Hi |
i - i - i - 11 I ' 11 i 1 ' -1 - - -1 p*"- i i -
9 0 85 8.0 7 5 7 0 65 6.0 5.5 50 4 5 40 3-5 3.0 ppm
13C/DEPT NMR 75 5 MHz
( 5 ) 7647
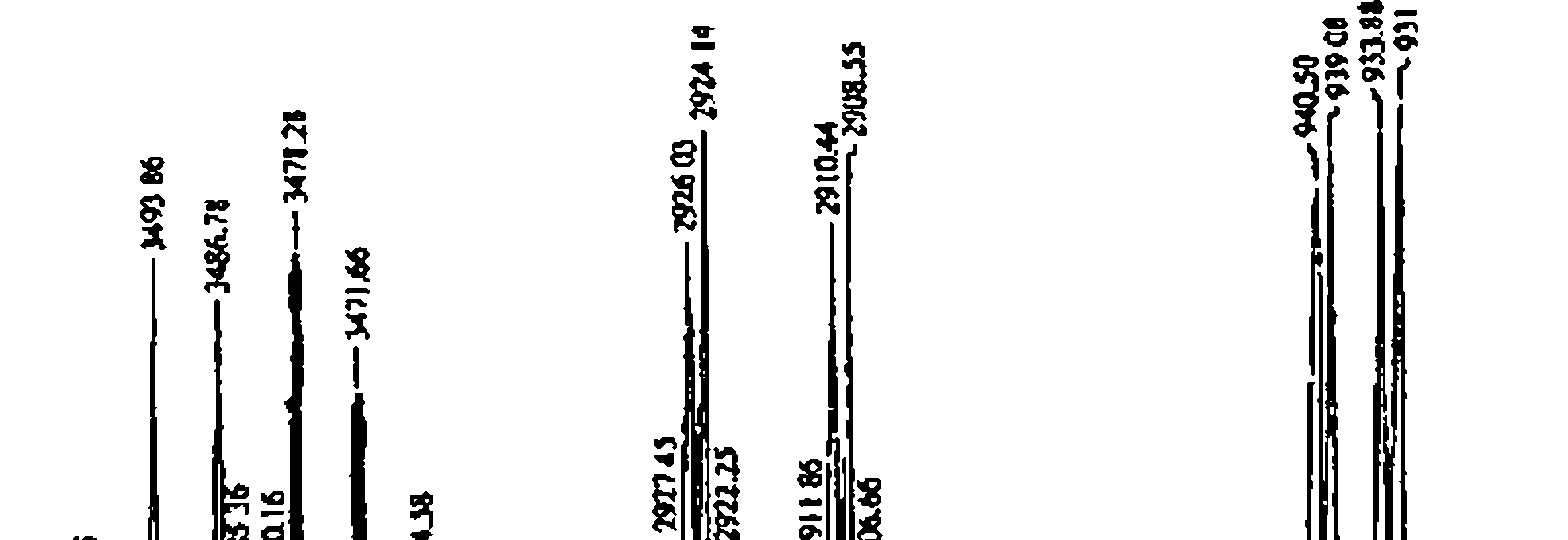
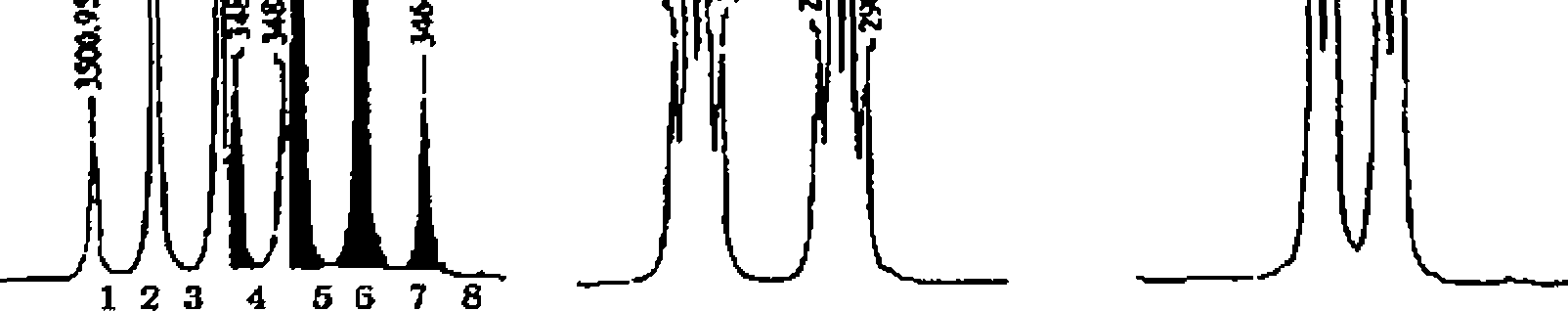
3 The molecular formula of an organic compound is C6H10O2 lH, 13C, COSY and HETCOR NMR spectra are given below In addition to this IR spectrum is also given below Find out the structure and assign all the peaks
lxii=ll
|
% Transmittance | 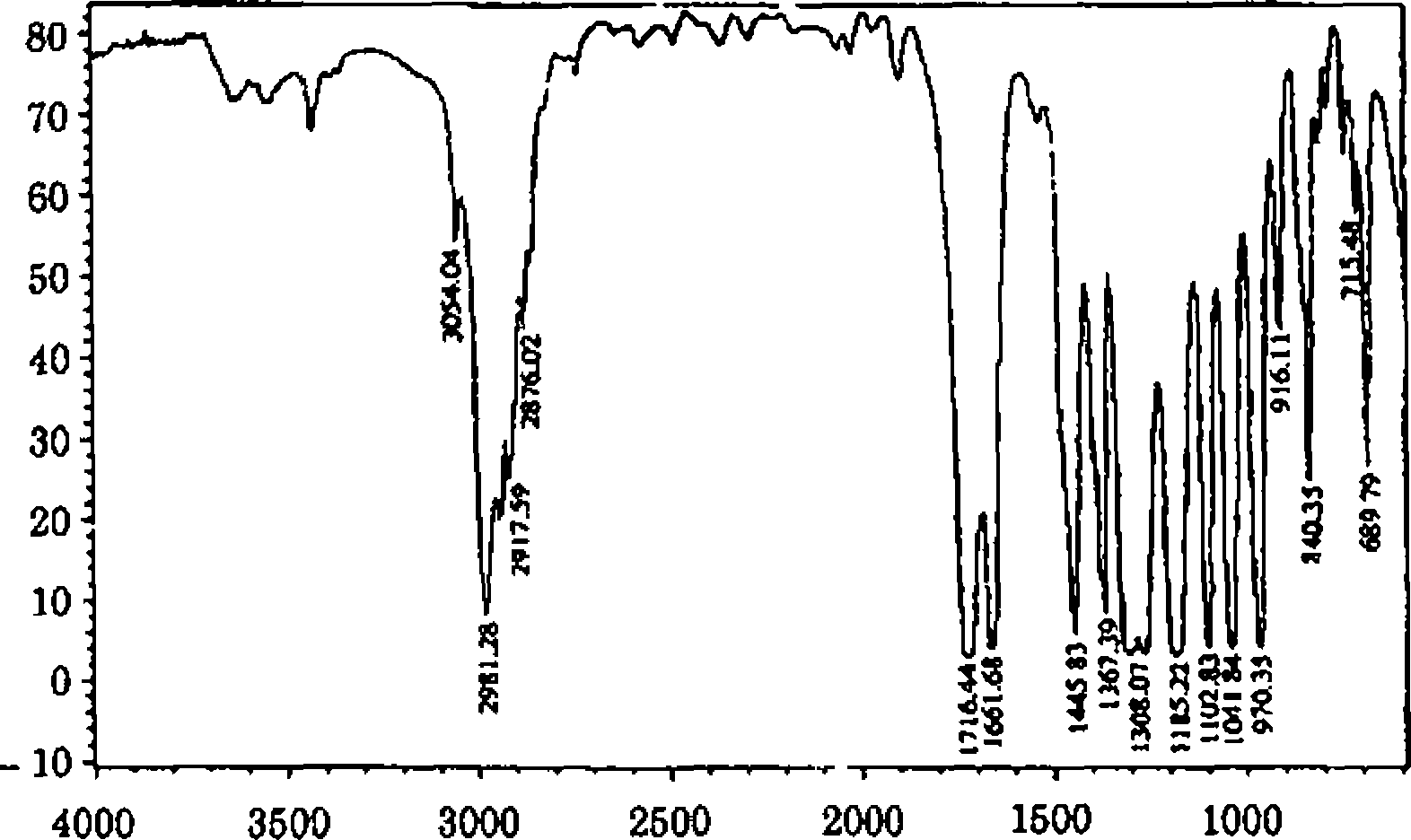 |
|
Wavenumbers (cml) |
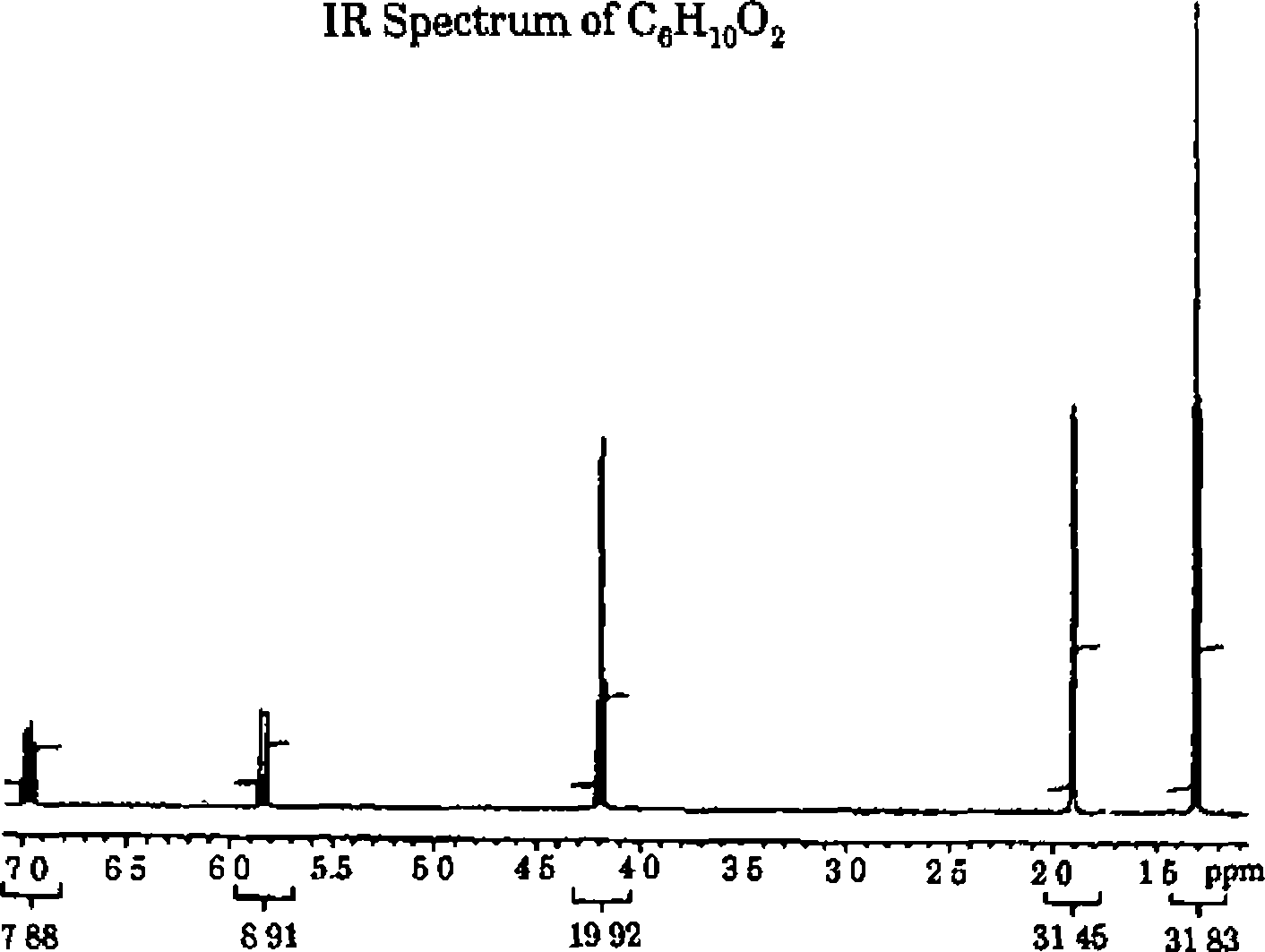 |
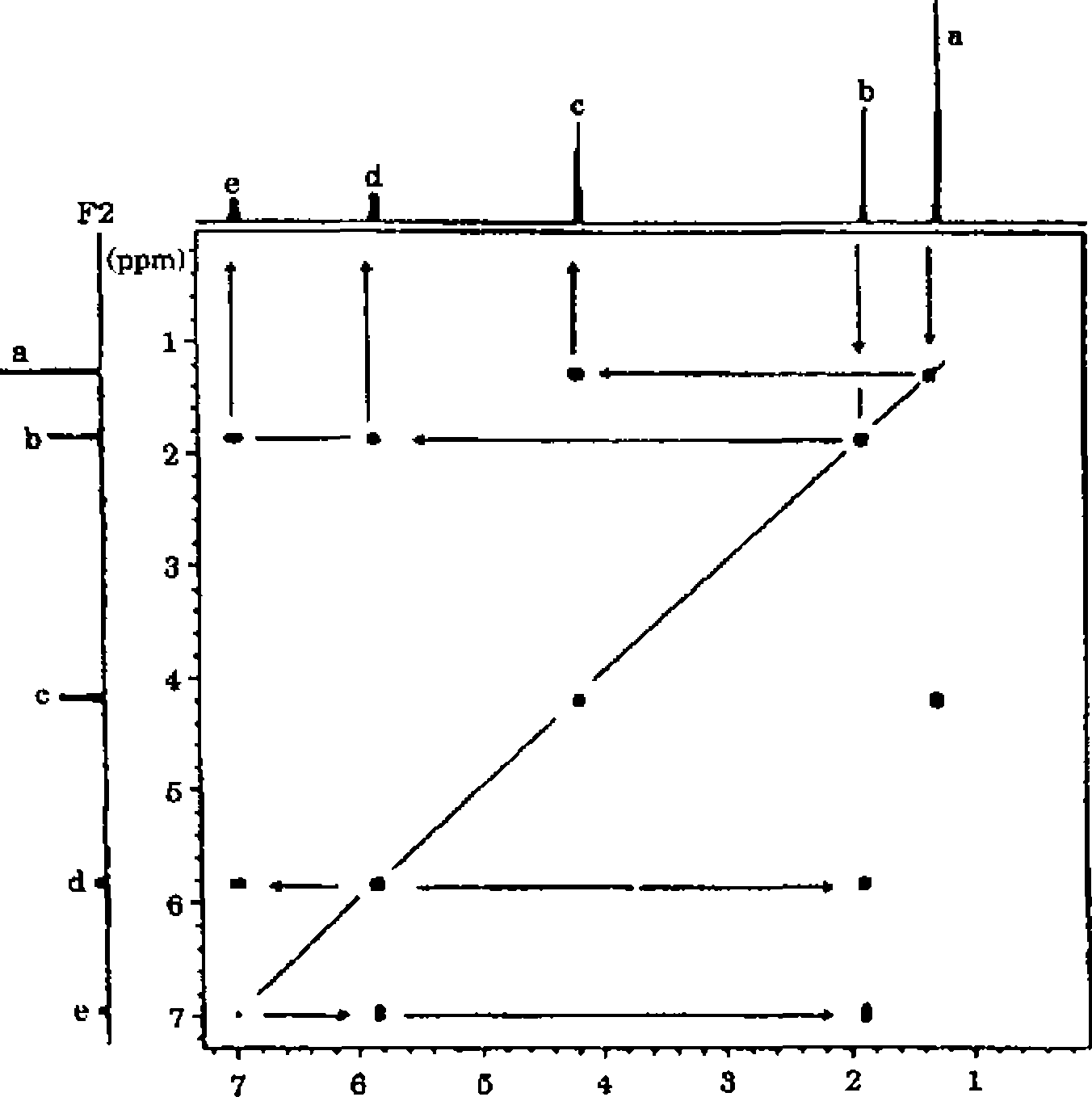
FI (ppm)
|
R |
 |
 |
|
-I--->-----1-' -----1-'-I-'->-1-r- >---(-1-1---1-J- 7 00 6 95 5 85 6 80 190 186 Expanded *H NMR spectrum of C6H10Oa |
COSY spectrum of C0H1()O2
F2
(ppm)
U
d-
7-k-
1_
' I.....I iipiM|Mii|iii i m n n n 11 m < n i'ih h * v
140 130 120 110 100 90 80 70 60 50 40 30 20 FI (ppm)
HETCOR spectrum of C6H10O2 Or
(a) Write short notes on any three of the following
3x3=9
(1) HSQC
(ii) HMBC (ni) ROESY Civ) FID
(b) Discuss the hyperfine splitting of diethyl ether radical 2x1=2
7647 ( 8 )
SECTION-B Answer three questions in all Question No 4 is compulsory.
1 (a) Discuss the mechanism of
(i) Hoch Campbell reaction (n) Davis Pizzini reaction
(b) Discuss the use of the azalactone route L-DOPA
11
2 (a) How can 1,3-dipolar cycloaddition be used for the
synthesis of isoxazoles
(b) What is Boulton-Katritzky rearrangement? Identify the product of the reaction of benzofuroxan with ethyl acetoacetate and morphohne Discuss the mechanism of this reaction 11
3 (a) Discuss a synthesis of Serotonin
(b) What are anomenc effect' and double anomeric effect? Predict the most favourable conformations for the two compounds given below
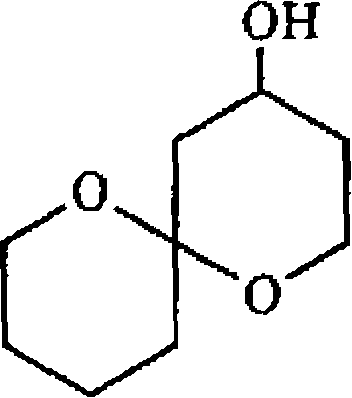
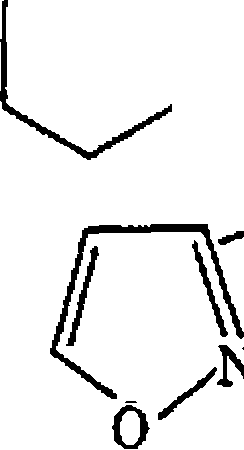
(i) ()
NHPh
=) 1 S
Explain the reaction and name the product
(d) How can Valium be synthesised? 11
( 9 ) 7647
4 (a) Discuss the limitations of the Corey Tramontano synthesis of methoxatin How can these be overcome9
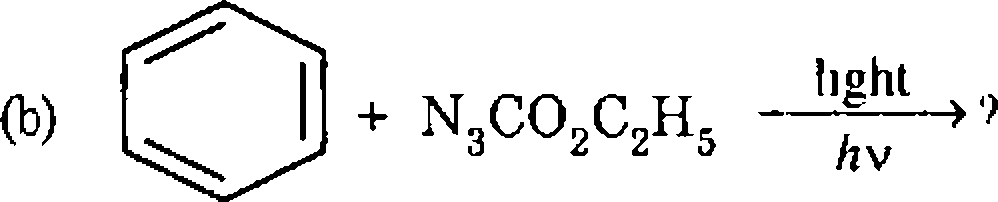
(c) Discuss the Corey-Chaykovsky reaction using both dimethyl sulphide and dimethyl sulphoxide
(d) How can the following be named systematically
cGI
Nh
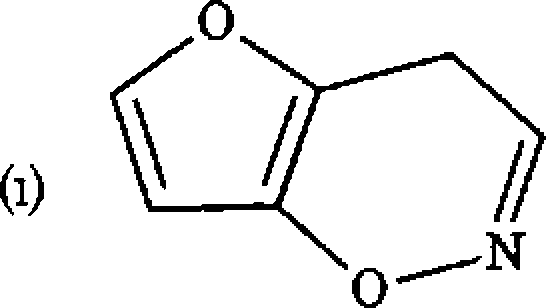
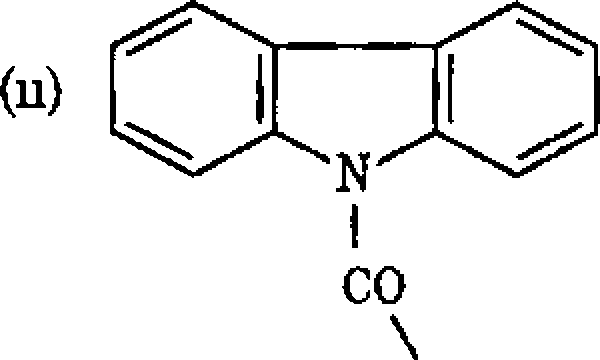
 |
NO, |
CH2NHN-
(e) Put down the chemical structure for (i) Trans-l,2-dimethyl-3-phenyl aziridine (n) 4H-[l,3]-Thiazino [3,4,-a] azepine
13
'"I........I.........I.........1"' y<'.-1.........J.......I"
190 ISO 170 160 150 140 130 120 110 100 90
|
Attachment: |
| Earning: Approval pending. |
