Manipal University 2007-2nd Sem B.E Mechanical Engineering I(MECHANICAL ENGG.) END SEMESTER (MAKE-UP) - Question Paper
III SEM. B.E. (MECHANICAL ENGG.) DEGREE END SEMESTER (MAKE-UP) exam JAN.2007
SUBJECT : BASIC ENGINEERING THERMODYNAMICS (MEE-201)
III SEM. B.E. (MECHANICAL ENGG.) DEGREE END SEMESTER (MAKE-UP)
|
Reg.No. |


MANIPAL INSTITUTE OF TECHNOLOGY
(A Constituent Institute of MAHE -Deemed University) Manipal - 576 104
EXAMINATION JAN.2007
SUBJECT : BASIC ENGINEERING THERMODYNAMICS (MEE-201)
REVISED CREDIT SYSTEM ( 05/01/2007)
Time: 3 Hours. MAX.MARKS: 50
Instructions to Candidates:
Answer ANY FIVE FULL questions.
Use of thermodynamics hand book and steam table are permitted.
Missing data can be assumed appropriately.
1A) Define the following:
i) Pure substance
ii) Dead state
iii) Second law efficiency
iv) Available and unavailable energy
v) Partial volume in a mixture of gases.
(03)
IB) Write a note on absolute thermodynamic temperature scale. (03)
IC) Atmospheric air is sucked in an internal combustion engine. The pressure, temperature and volume of air after suction are 1 bar, 75C and 800 cm3. The air is then compressed to 15 bar and 1/8th of its volume. Heat of combustion of fuel is then added to air at constant volume until its pressure is 50 bar. Determine
i) Index of compression
ii) Change of entropy during each process.
iii) Determine if there is any heat exchange between air and cylinder walls during compression. State the direction of heat flow. Show the processes on P-V and T-S diagram. (04)
2A) Derive the four Maxwells equations. (03)
2B) 400 kJ of heat from a large heat source at 1000 K is supplied to 2 kg of air initially at 2 bar and 350 K at constant pressure. Find the loss in available energy due to heat transfer. Take surrounding temperature as 300 K. (03)
2C) State and prove inequality of clausius. (04)
3A) Show that entropy change between state 1 and 2 in a polytropic process is given by
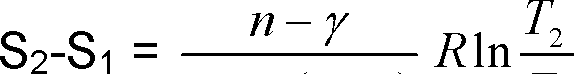 |
|
Y-1 (n -1) Ti |
3B) Draw the phase equilibrium diagram for a pure substance on P-T
coordinate and explain the diagram.
(03)
(04)
3C) Determine the mass density of steam at 100 bar and 600C. Using
i) Steam table
ii) Perfect gas equation.
iii) Vander waals equation.
4A) The following data were obtained with separating and throttling calorimeter.
Pressure in pipeline = 1.5 MPa Pressure after throttling = 0.1 MPa Temperature after throttling = 110C.
Water separated in the separating calorimeter = 0.15 kg.
Steam condensed after throttling = 3.25 kg.
Assuming specific heat of superheated steam = 2.1 KJ/Kg.K. Find the quality of the steam in the main. (03)
4B) 2 kg of wet steam at 10 bar is heated at constant volume and the heating process passes through the critical point. The pressure after heating is 100 bar. Find the mass of dry vapour before and after heating. (03)
4C) Express the Vander Walls constant in terms of critical properties and
show that the compressibility factor Zc= 3/8. (04)
5A) Show that the efficiency of reversible engine is Greater than that of irreversible engine operating between a given constant temperature source and sink. (03)
5B) The temperature scale of certain thermometer is given by the relation.
T = a lnp +b
Where a and b are constants p is thermometric property of the fluid in the thermometer. If at ice point and steam point the thermometric property and found to be 1.65 and 7.39 respectively, what will be the temperature corresponding
P = 3.5 on Celsius scale.
(03)
5C) 1 kg of ice at -5C is exposed to atmosphere which is at 25C. The ice melts and comes into thermal equilibrium with atmosphere.
i) Determine the entropy change of the universe.
ii) What is the minimum amount of work necessary to convert water back into ice at -5C?
Take specific heat of ice = 2.093 KJ/Kg.K.
Latent heat of fusion of ice = 333.33 kJ/Kg.
Specific heat of water = 4.187 KJ/Kg.K. (04)
6A) Show that on a P-V diagram two reversible adiabatic paths cannot
intersect each other. (03)
Y n
Y 1
x
(03)
Show that heat transfer during polytropic process is given by Work done. Derive it from fundamentals.
6C) A mixture of ideal gas consists of 4 kg of nitrogen and 6 kg of carbon dioxide at a pressure of 4 bar and 20C. Find
i) The mole fraction of each constituent.
ii) The molecular weight of the mixture.
iii) The partial pressures and partial volumes.
iv) The volume and density of the mixture. (04)
(MEE-201) Page 3 of 3
|
Attachment: |
| Earning: Approval pending. |
