Gujarat University 2007 B.E Chemical Engineering thermodyanamics 2 - Question Paper
GUJARAT UNIVERSITY
B. E. Sem VI (Chem) exam
Chem. Engg. Thermodynamics-II
/
Assume suitable data if require.
Figures to the right Indicate full iti;un>
Use oTK-Charts is allowed.
______________SECTION - I
1 Answer the follow inc
/ A system comprised of four particles has four energy levels with relative -energy levels of 0. 1. 2, 3. The total energy of the system is 4 and the degeneracy of the four levels is 4, 4, 3, 3 respectively. .Determine the thermodynamic probability for each of the possible disuiuuiiuiis assuming Boltzmann's statistics.
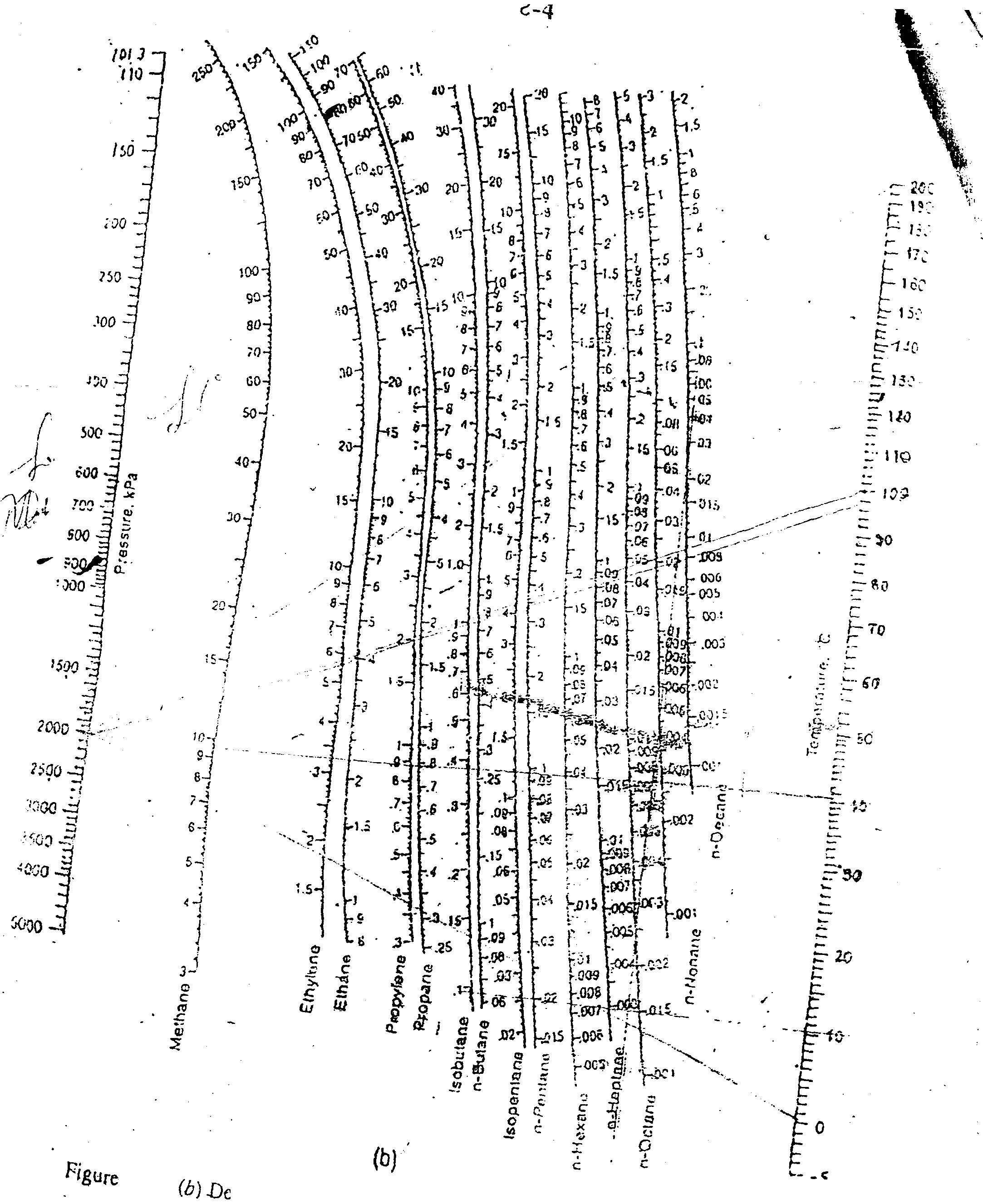
[1(b) A retineiy gas contains 10% methane, 10% ethylene* 20% propylene, - 35% propane and 25% of n-butane by volume. It is to be compressed and liquefied in water-cooled condenser. If the minimum temperature attainable in the condenser is 20 C, to what pressure should the stream be compressed to achieve the total condensation?
GUJARAT UNIVERSITY -
B. E. Sein VI (Chtin.) Examination Chm. Engg. Thermodvnamics-II
Time : 3 Hours Max. Marks : 100
Answer to the tw o sections must be written in separate answer books.
All notations have conventional meaning.
For A and B are miscible liquids,
GE/RT = 0.1x,x2 AG29s = -I000J

v-aicuiaie.
1. Equilibrium constant kK and Equilibrium composition of A and B.
2. Sf system is assumed ideal, calculatc the error in equilibrium composition of A.
CR
-2 Attempt the following 16
Write in brief on vanous methods for evaluating the equilibrium constant ' f_(b) Derive tbs relationship between Equilibrium constant and standard Gibbs free energy change.
3 (a)"~ttempt any two short notes of the following 10 Group Contribution Methods.
Equilibrium conversion charts.
Area tests.
(b) Attempt any one short notes of the following 06
Vant Koff equilibrium box.
T-x,y diagram for partially miscible system.
SECTION -n
4 Answer the following .
' Discuss the various methods to cvajuate the fugacity and fugacity 08
coefficient of pure component? fbYi Estimate the ftioacitv of isobutylene as a gas al 280C and 20 bar. 10
The required property values for pure isobotylenc are:
|
CO |
Tt |
! Pc __ |
|
0.194 |
417.9 K |
1 40.00 bar |
I Friday, 1st June, 2007]
Instructions : (1)
(2)
(3)
(4)
(5)
 |
|
11) |
pj i y Tt
XX
'U )
08
.*aAv"
5 (a) Prepare plots of (f vs. P) and of vs. P) for isobutane at 40 C for the 08 pressure range fifmi 0 to 10 bar.
At 40 C the vapor pressure of isobutane is 5.28 bar.
Data: The molar volume of saturated liquid is given by Rackett equation.
{i-T'f
The required property values for pure isobutane:
V "
\
|
i G) | Tc |
XT |
! Vc |
IzT ! ...1 _ J |
|
| 0.181 jOS.l K |
36.48 bar |
| 262.7 cm'Vmol |
lU82| |
1(b) Write a brief note on retrograde condensation and its application.
OR
5~~-(a)j Write a brief note on Margules equation and Wilson equa*h5?i used for 08 non-ideai solutions. ~~
(b) Omiine the method to evaluate the bubble temperature and dew point ,08 temperature for system exhibiting VLE for ideal and nonideal systems.
6 Answer the following
Perry Oabiel3-l) quotes the following T~x.y data for Acetone (I) and 16 methanol (2) at 101.3 kPa total pressure. Estimate the Van Laar constants ApatulA-,,.
|
i k i_I |
Vi |
T / r i i V/ |
V* i |
- y | |
|
64.65 |
o.o |
0.0 |
55.78 |
0.578 |
0.631 1 |
|
61.78 |
0.091 |
0.177 |
55.41 |
0,687 |
0.707 I |
|
59.60 |
C.190 |
0.312 |
55.29 |
0.756 |
0.760 1 |
|
r 58.14 H |
0.288 |
0.412 |
55.37 |
r 0.840 |
0.829 |
|
56.96 |
0.401 |
0.505 |
55.54 |
0.895 |
0.880 |
|
56.22 |
0.501 |
0.579 |
55.92 |
' 0.954 |
0.946 |
|
56.21 |
j 1.000 |
1.000 |
OR
A binaryjsystem of 1 and 2 consists of vapor and liquid phases in equilibrium at temperature T. The overall mole fraction of 1 in the system is 0.6 for which - 32.27kPa, P2sat= 73.14kPa and GE/ RT = 0.95 x5x2. Making relevant assumptions, determine the rs
. of the pressures for which the system cxisLasisro phases at given'temnergtnre T and overall jnole faction. Also determine ihe pressure and the composition of the azcoirope at temperature T, ifformed=
yfi- = v.z)
bit
r )
'ik
08
 |
|
2 jd 1 o |
1.3-
116
-j
-*1 200 -1 -1 3
250 300 -j
50-1
JOH
08
"flS
30.
400
500
600 d 700
00
aoo-j v. 10C0 '
r
i *
F-
f~
t
L
L
t
J-
r
i-
i~
1500 ~j
3000
6000
<0
5
t--
(fl) be Prit;>r chan >- .-
L,
Hgurc
' hydrocarbons, tow Kmpaaiure
P. T. O.
\
|
M |
 |
|
' Priesirr Mart *]/??! . |
for hyd,,

|
Attachment: |
| Earning: Approval pending. |
