Deemed University 2010 B.Tech Mechanical Engineering University: Lingayas University Term: III Title of the : Thermodynamics - Question Paper
Roll No. ..
Lingayas University, Faridabad
Examination May, 2010
Course: B.Tech. Year: IInd
Semester: III Paper Code: ME-201E
Subject: Thermodynamics
[Time: 3 Hours] [Max. Marks: 100]
![]()
Before answering the question, candidate should ensure that they have been supplied the correct and complete question paper. No complaint in this regard, will be entertained after examination.
![]()
Note: Attempt any five questions. Assume suitable missing data, if any
Q-1. (a) Derive the expression for the displacement work transfer during an adiabatic process. (8)
(b) What is control volume. Differentiate between Lagrangian and Eulers approach of analysis of control volume. (6)
(c) What do you mean by Homogeneous and Hetrogeneous system? (6)
Q-2. (a) What is free expansion process. Explain in brief with Sketch. (8)
(b) In a steady flow apparatus, 135 kj of work is done by each kg of fluid. The specific volume of fluid, pressure and velocity at the inlet are 0.37 m3/kg, 600 kPa, and 16 m/s. The inlet is 32m above the floor and discharge pipe is at floor level. The discharge condition are 0.62 m3/kg, 100 kpa and 270 m/s. The total heat loss between the inlet and discharge is 9 kj/kg of fluid. In flowing through this apparatus, does the specific internal energy increase or decrease and by how much. (12)
Q-3. (a) What is carnot cycle? What are its different process. (8)
(b) A heat engine is used to drive a heat pump. The heat transfer from engine and from the heat pump are use to heat the water circulating through the radiaters of a building. The efficiency of heat engine is 27% and the COP of heat pump is 4. Evaluate the ratio of heat transfer to the circulating water the heat transfer to the heat engine. (12)
Q-4. (a) Three identical finite bodies of constant heat capacity are at temperatures 400, 400 and 100k, if no work or heat is supplied from outside what is the highest temperature to which any one of the bodies can be raised by the operation of heat engines or refrigerators. (10)
(b) Why is the entropy increase of an isolated system a measure of the extent of irreversibility of the process undergone by the system? (10)
Q-5. (a) What is throttling calorimeter, explain its working with help of neat sketch. (8)
(b) Steam of 65 bar, 400oC leaves the boiler to enter a steam turbine fitted with a throttle governor. At a reduced load as the governor take action the pressure of steam reduced to 59 bar by throttling before it is admitted to the turbine. Evaluate the availability of steam before and after the throttling process. (12)
Q-6. (a) What is vander waals equation and give the limitation of its equation.
(8)
(b) A vessel of capacity 3m3 certain 1 kg mole
of N2 at 90oC. Calculate pressure and specific volume of
gas. If ![]() = 1.4
evaluate the value of cp and cv. (12)
= 1.4
evaluate the value of cp and cv. (12)
Q-7. (a) Derive the Maxwell relation. (12)
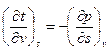
(b) Explain co-efficient of expansion and compression briefly. (8)
Q-8. Write short notes on following:
(a) Macroscopic and macroscopic approaches. (8)
(b) Third law of thermo dynamics. (6)
(c) Dryness and wetness fraction of steam. (6)
Roll No. ..
Lingayas University, Faridabad
Examination December, 2009
Course: B.Tech. Year: IInd
Semester: III Paper Code: ME-201E
Subject: Thermodynamics
[Time: 3 Hours] [Max. Marks: 100]
![]()
Before answering the question, candidate should ensure that they have been supplied the correct and complete question paper. No complaint in this regard, will be entertained after examination.
![]()
Note: Attempt any five questions. Assume suitable missing data, if any
Q-1. (a) Derive the expression for the displacement work transfer during an adiabatic process. (8)
(b) What is control volume. Differentiate between Lagrangian and Eulers approach of analysis of control volume. (6)
(c) What do you mean by Homogeneous and Hetrogeneous system? (6)
Q-2. (a) What is free expansion process. Explain in brief with Sketch. (8)
(b) In a steady flow apparatus, 135 kj of work is done by each kg of fluid. The specific volume of fluid, pressure and velocity at the inlet are 0.37 m3/kg, 600 kPa, and 16 m/s. The inlet is 32m above the floor and discharge pipe is at floor level. The discharge condition are 0.62 m3/kg, 100 kpa and 270 m/s. The total heat loss between the inlet and discharge is 9 kj/kg of fluid. In flowing through this apparatus, does the specific internal energy increase or decrease and by how much. (12)
Q-3. (a) What is carnot cycle? What are its different process. (8)
(b) A heat engine is used to drive a heat pump. The heat transfer from engine and from the heat pump are use to heat the water circulating through the radiaters of a building. The efficiency of heat engine is 27% and the COP of heat pump is 4. Evaluate the ratio of heat transfer to the circulating water the heat transfer to the heat engine. (12)
Q-4. (a) Three identical finite bodies of constant heat capacity are at temperatures 400, 400 and 100k, if no work or heat is supplied from outside what is the highest temperature to which any one of the bodies can be raised by the operation of heat engines or refrigerators. (10)
(b) Why is the entropy increase of an isolated system a measure of the extent of irreversibility of the process undergone by the system? (10)
Q-5. (a) What is throttling calorimeter, explain its working with help of neat sketch. (8)
(b) Steam of 65 bar, 400oC leaves the boiler to enter a steam turbine fitted with a throttle governor. At a reduced load as the governor take action the pressure of steam reduced to 59 bar by throttling before it is admitted to the turbine. Evaluate the availability of steam before and after the throttling process. (12)
Q-6. (a) What is vander waals equation and give the limitation of its equation.
(8)
(b) A vessel of capacity 3m3 certain 1 kg mole
of N2 at 90oC. Calculate pressure and specific volume
of gas. If ![]() = 1.4
evaluate the value of cp and cv. (12)
= 1.4
evaluate the value of cp and cv. (12)
Q-7. (a) Derive the Maxwell relation. (12)
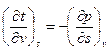
(b) Explain co-efficient of expansion and compression briefly. (8)
Q-8. Write short notes on following:
(a) Macroscopic and macroscopic approaches. (8)
(b) Third law of thermo dynamics. (6)
(c) Dryness and wetness fraction of steam. (6)
| Earning: Approval pending. |
