Veer Narmad South Gujarat University 2011-2nd Year B.Sc Chemistry SB-0711 Psysical : - 5 - Question Paper
Time : 3 Hours] [Total Marks : 70
Second Year B. Sc. Examination
March/April - 2011
Physical Chemistry : Paper - V
SB-0711
nLal PuiKhloft [cPMi SlMtfl.
Fillup strictly the details of signs on your answer book.
Name of the Examination :
S. Y. B. Sc.
Name of the Subject:
Physical Chemistry : Paper - 5
Student's Signature
|
-Subject Code No.: |
|
-Section No. (1,2,.....): Nil |
(0 Ul T. dl HHl ntVHld d.
(3) ihY ?Hd SRR (3tR ?Hl[ct %ll*t C-tHL M WiO. "Hit'll M& %|R d.
*1 -Mdldl ZhHi WH ?HLUL : *IH
(*0 JUSlieM l4 860 AG= -9.8KJ d. dl ddl
h'lOl (pv) i6H llMTl.
(0 % M U&HKL dC-W 0.2 dl ddl AG-(I *i$ll +ve
I -ve snii ?
(3) (3*Hl?llH U&HL Hia Inkpvsj dl Rl.
M 5d (M-ft
(m) HL'iCldL llR-ft &Hd 25C dlUHld kefl. d ?
(?) <l6diM 5HLUL. (0) l6Rl*ldl HlUdldl d>L =Mddl GHHH C-tHL (<0 PUWSI S$L.
SB-0711] 1 [Contd...
(fc) WKHl'Yd-ft C-IHL
(<lo) Hh H H&HI C-IHL %HL kCKl 5HHVUHI H< (3&H Hd 5HHVUHI 61H.
(*1*1) =HLHL C-IHL
(<u) - R OdHH C-lHL
(*13) IMh Hdl H&HI Vll &HH %U-4Hl H< &Kdl HLSl/teHL HHIH dl H&HI ?R ?>RUdl 5HH C-tHL (lY) -flaldlHl(l &HI U31h2 *llH*l ? *11 HIS
HkeU WLHl fcUIrt HlHld USWHl fcUIrt *di HHR *11 HIS 61H d ?
1 (h) ofl*0> H< 6<mi<&0Ml irflMn H=4dl d$LHdl C-IHL Y
=*llctL
{h) *Wa dlHHld dX-M HWlh ?Hd (M d$LHd Hdl Y
-ftHWCl. (nVa &HdlH Htal).
(H) H&Hldl 5d (M d$LHd ?Hd ddl dlHHld Hdl Y
Ulhd ofl*0> <k-H6lC-Stf aifod hiI.
H*l<U
(h) 34.0hlH1 WiPldl d ? M, AG 5Hd Kp -ft %isLKl LfcotHl Y %LH*4a<Cl.
U) fcHKl Mh tmi-i (3rMd IM| 100.128C d. % = 0.512 3 ?Hd K f = 1.86 K/m 61H dl $Ul5l 6R&I 5>llHl.
3 (?H) HIH HlUdMl *UH& <U6$dlI*lRlH HlHddl $IHU C-tHL Y 5HL HlHdlHl tfl hY'\?$ Ciql Hi d H'SlHL.
H*l<U
(*l) C/dl $U<*ldl plR dl LHLHL lteRl*ldl OdHH-ft Y
(3HHll>LdL %IH*1<1, &d H'sRl.
SB-0711] 2 [Contd...
eHll M Wdl <Hd OHHHL C-tHL Y H*l<U
fealdL UHil q.<iWl dHLft HldL OHmI ttH'SA'fl. Y 20 C dLUHLd HL'iCl ?Hd g6r sUldl fal>L q.L6Sdl ?HH 3
(H)
(*0
U)
1.519X10-6 1.576X10-6 M_1 &. ?Hdd Hd, KN03,KBr ?Hd M?3 dL sUUdl d<miMl ?HH 181.3,187.4 ?Hd 121.0 &. dL ,4gBr SLLdL $U<*ldL LLR ?LLHL.
X (?h) ?HdL HL'iCl ?Hd <hFoM ldi Old.'iL HL8 HLH X
(*l) MddL 6L&L?SH*Ld foLL C-t (3&U HL8dL HPuilH'iiq.lS. Y ttH'SA'fl.
(h) 1 te 'tfeG.H %HL 5gm 5HL*Md &, dd 50 Mk. CS2 3 sU=lM [dMd hl&l dC-id &HH 'tfC-tk saLHL *Hlil *6dL 5HL*MddL mi LISLL. CS2 d*U HL'iCl Ut /2 dL K,} = 90.
H (?H) ci'iha & ? ddL ?. UUldl dLH d*U dHdl *LL*i Y *UILH<3. [rt, dlC-i.<Hl*S ?Hd Eddl DsHdL ClHl dHL *l& WiWldl $M dLH ?HLUL.
M %lHLd [Hid-ft U&HL * 5>RIL Hl<fl. Y
(<h) ML?LHdLdL HLL&ddL GdHH C-tHL (M d*U dM Y MVan i*kHdi Hiadi ham c-ihl
H*l<U
Oh) d*U L* dLH C-tHL Y
U) M UfeHLdL anSGHHl Ldi 5>mq. A =*Hd JL&HL 3
5>RlUr kdL \HL 4xl013 S_1 d 1.155xlO3 S_1
9. dl AOOK dLHHLd feHL *Lfd Ea ill Hi.
R = 8.314 JiC-1 wo/-1
e (?h) 31Hd HSha U* dLH C-IHL. iUd q.<ilU2 5H2<H ? Y
H*l<U
(?h) HQIH'SL cL'ihai & : Y
VjJ+1 = 2/y (./ +1) 677? 1 dL ddL hM <HH C-i<Hl*S &d *LLHLH
d %lHWil. HL HL U&Hftd & ? HQIH'SL q.<ilU2 Hmi HlMl HLa <H *L*dL & ?
(h) (l) /i? HSha HLa P, Q, ?Hd i? ?LLHL?Hl 6 d 5HL[ct &fed Y *LHWil.
(0 CO HlMl XMdl 1.46xl0-46 kgm1 6LH dL ddl
UIH (3d[Vd aHL HLMl HkOlH'SL *LEd <HHL *LLH"l.
/? = 6.626xlO34Js
H*l<U
(<h) /i? qLHadl (3HHLl>LdL *Lfed* C-tHL Y
($) tfCM *jwi ?HLac-id 5HL[rl 2890 cm-1 &. dL dL Hl 3
Nm~l LLMl. H = \amu Cl = 35.5amu C = 3xlcPms~l
5HU N = 6.023 xlO23 SB-0711] 4 [Contd...
(2) All sub questions of Q. 1 are compulsory.
(3) Answer pointwise with proper figure.
(4) The no. on right indicates full marks.
1 Answer in brief :
15
(i) A system does total work of 860J, and its AG=-9.8kJ
calculate mechanical work done by it.
(ii) If equilibrium constant of a reaction is 0.2, predict sign
of AG.
(iii) Draw the plot of In Kp vs.l/T for endothermic reaction.
(iv) Define free energy.
(v) What is the value of ionic product of water at 25C?
(vi) Define transport number.
(vii) State Kohlraush's law of independent migration of ions.
(viii)What is distribution coefficient ?
(ix) Define hydrolysis.
(x) Write a chemical reaction in which reactants are in gaseous state and catalyst in solid state.
(xi) Define Einstein.
(xii) Write Grotthus Draper law.
(xiii) Give the unit of second order rate constant when time is measured in sec and concentration in terms mole/litre.
(xiv) Which molecule will give rotational spectrum and give the reason :
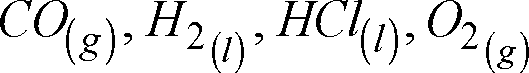
(xv) Why antistokes line has higher frequency than frequency of incident radiation ?
(a) Derive relation between equilibrium constant and free 4 energy change at constant temperature (van't Hoff reaction isotherm)
(b) How free energy change is related to its temperature 4 coefficient; derive Gibbs Helmohltz equation.
(b) What is spontaneity of a reaction ? Write criteria 4 for spontaneity in terms of AS, AG and Kp.
(c) The boiling point of aqueous solution of urea is 3 100.128C calculate its freezing point .
Kb = 0.512K/m, Kf = lMK/m
3 (a) State advantages of conductometric titration over 4
volumetric titrations. Discuss precautions taken in this titrations.
(a) Explain how Kohlrausch law can be used to determine 4 solubility product of AgCl.
(b) Discuss Hitorff experiment and conclusion drawn from 4 it.
(c) At 20C, specific conductivity of water and AgBr soln. 3 are 1.519x 10-6 and 1.576xl0-6 mho/cm respectively.
At infinite dilution equivalent conductance of KN03, KBr
and AgNO soln. are 181.3, 187.4 and 121.0 mho respectively. Calculate solubility product of AgBr.
4 (a) Derive equation for distribution of benzoic acid 4
between water and benzene.
(a) Explain adsorption theory of catalysis taking 4 hydrogenation of ethene as an example.
(b) Differentiate between physical and chemical 4 adsorption.
(b) Discuss benzenoid-quinonoid theory of acid base 4 indicators with proper structures of atlcast two indicators.
(c) An aqueous soln. contains 5gm. of iodine in 1 liter 3 is extracted with 50 ml. carbondisulphide;calculate the amount of iodine remaining in aqueous layer after extraction. KD of iodine between carbondisulphide and water=90.
5 (a) What is electromagnetic apectrum ? Give the names 4
of region, frequency values wavelength values and energies values and spectroscopy involved in different region of spectrum.
(a) Derive the equation of rate constant from collision 4 theory.
(b) State Einstein law of photochemical equivalence; give 4 reasons for high and low quantum efficieny.
(c) In the Arrhenius equation for a certain reaction, the 3 value of frequency factor A and rate constant k are 4xl013s-1 and 1.155xl0_3s_1 respectively, calculate Ea
at 400K (R=8.314 JK1 mol'1).
6 (a) Explain briefly vibrational spectra and Raman 4
spectra.
Vjj+i = 2B{J + l)cm~l. Explain how bond length can be
obtained using this equation. Which transitions are spectroscopically forbidden ? What are the two important conditions of a molecule to show rotational spectrum ?
(b) (i) What are P, Q and R branches of IR spectrum? 4 Explain figure.
(ii) The moments of inertia of CO molecule is
1.46x10-46kg m2. Calculate rotational energy in joules for first excited state. h=6.626x 10-34JS.
OR
(b) Discuss applications of IR spectroscopy.
(c) The fundamental vibrational frequency of HC1 is 3 2890 cm-1. Calculate force constant of this molecule in Nm-1. H=lamu, Cl=35.5amu, c=3xl08ms_1 Avogadro
no .= 6.023xl023.
SB-0711] 8 [2500]
(a) Compare Gibbs' and Helmholtz's work function.
OR
|
Attachment: |
| Earning: Approval pending. |
