Veer Narmad South Gujarat University 2011-2nd Year B.Pharm SB-1153 PH-203 Pharmaceutical Chemistry - 2 a - Question Paper
SB-1153
Second Year B. Pharm. Examination March / April - 2011 PH-203 : Pharmaceutical Chemistry - II
(Organic)
Time : 3 Hours]
[Total Marks : 70
""'N Seat No.:
6silq<3i Puunki(l faaim SuwiA u*
Fillup strictly the details of signs on your answer book.
Name of the Examination :
S. Y. B.PHARM.
Name of the Subject:
PH-203 : PHARMACEUTICAL CHEMISTRY - 2
|
-Subject Code No. |
|
1&2 |
Student's Signature
Attempt any ELEVEN of the following :
11
(a) Write electronic configuration for chlorine (atomic number 17) and bromine (atomic number 35)
(b) Which of the following has
(i) The most polar bond
(ii) The least polar bond Nal, LiBr, KC1, Cl2
(c) Which is strong acid - acetic acid or fluroacetic acid ? Why?
(d) Why NH3 has 1.46D dipole moment but NF3 has 0.26 D ?
(e) Why o-nitro phenol have much lower boiling point and much lower water solubility than their meta and para isomer ?
(f) Why aromatic amines are weaker base than aliphatic amines ?
(h) Show the orbital geometry in sp, sp2 and sp1 hybridization with example.
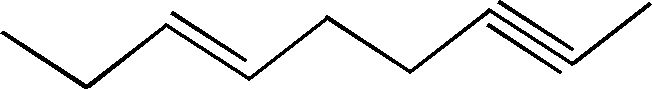
(g) What is the hybridization state of each of the carbon atoms in following compound ?
(i) Explain why phenol is more acedic than alcohol.
(j) Draw the conjugate acid of each of the following :
(i) NH3 (ii) Cl-
(iii) OH- (iv) H20
(k) Draw a compound that contain only carbon and hydrogen atoms and that has 4 sp3 hybridized carbon.
(1) HC1 is weaker acid than HBr. Why does ClCH2COOH a stronger acid than BrCH2COOH ? - Explain.
(m) Draw three constitutional isomers with molecular formula
c3h8o.
(n) Enlist the conditions necessary for resonance.
2 Attempt any four : 12
(a) Write down the preparation and reaction of Ether.
(b) Explain El and E2 mechanism with suitable example.
(c) Explain Fries rearrangement and Kolbe reaction with mechanism.
(d) Discuss Hydroboration-Oxidation reaction in detail.
(e) Write down the preparation and reaction of Alkane.
(f) Why electron releasing group on benzene ring are ortho/ para directors in electrophilic aromatic substitution ?
4 Attempt any five of the following : 10
(a) Draw perspective formulas for the following :
(R)-2-butanol II) (2s, 3R)-3-Chloro-2-pentanol
(b) Explain the following terms with suitable example :
(i) Enantiotopic hydrogen
(ii) Homotopic hydrogen.
(c) Draw all possible stereoisomers for 3-chloro-2-butanol.
(d) Define and classify stereoisomerism.
(e) Which of the following isomers differ in constitution and which in configuration ?
(i) (-)-lactic acid and (+)-lactic acid.
(ii) 1-chloropropene and 2-chloropropene
(f) List the substituents in each of the following set in order of priority from highest to the lowest :
(i) -Cl, -SH, -OH, -H
(ii) -F, -S-CH3, -HC=0, -CH3
5 Attempt any four : 10
(a) Give two methods for synthesis of imidazoles.
(b) Classify terpenoids on the basis of number of carbons and explain isoprene rule with example.
(c) Give chemical properties of amino acids.
(d) Give two methods for synthesis of pyrroles.
(e) Give two methods for synthesis of furans.
(f) Write classification of lipids.
6 Attempt any five : 15
(a) Define glycosides and give the classification.
(b) Give brief account of chemistry and medicinal uses of taxol derivatives.
(c) Write short note on sigmatropic reaction.
(d) Write methods of preparation of alkyl halides.
(e) Write reactions of phenols.
(f) Explain in detail molecular orbital theory.
(g) Write short note on carbocation and carbanions.
SB-1153] 3 [ 500 ]
Attempt any three : 12
(a) Explain cycloaddition reaction.
(b) Write a note on Hybridization.
(c) Explain with example aldol condensation reaction.
(d) Discuss with mechanism Reformatsky reaction to synthesize a, (3 unsaturated carbonyl compounds.
(e) Explain the cumene process for industrial phenol production.
|
Attachment: |
| Earning: Approval pending. |
