Aligarh Muslim University (AMU) 2011 B.Sc Physics Thermal and statistical - Question Paper
4481
2010-2011 B.Sc. (HONS.) (PART - III) EXAMINATION (PHYSICS)
THERMAL AND STATISTICAL PHYSICS
(PH-314)
Maximum Marks : 40 Duration: Three Hours
Note: Answer all questions.
. t.
<,"
1.(a) Starting from the basic assumptions, derive the Van der Waals equation of state. [3+3] Show that the constants of the equation can be expressed in terms of critical constants.
(b) Plot the typical variation of intermolecular forces as a function of separation [1] between molecules and discuss the features of this plot.
OR
l'.(a) Discuss the phenomenon of viscosity in gases and hence derive an expression for the [4+1] coefficient of viscosity (r)) of a gas. Discuss the dependence of r| on the temperature of gas.
(b) Define macroscopic collision cross-section of molecules. [2]
2.(a) State the first law of thermodynamics. Write the mathematical form of this law and [4] explain its significance.
(b) Calculate the amount of work done when one gram mole of a perfect gas expands [3] isothermally at 127C, increasing the volume to four times the original volume (Given: R=8.3 Joules/degree/mole).
3.(a) State and prove the Carnots theorem. [4]
(b) Obtain an expression for the entropy of an ideal gas in terms of pressure and [2] temperature.
OR
Discuss the entropy of mixture of two gases and hence explain Gibbss paradox. [4J
(b) Sketch the T-S diagrams for isentropic, isochoric, adiabatic and isothermal [2] processes.
.(aj Calculate under what pressure ice freezes at 272 K, if the change in specific volume [4] ] when 1 Kg of water freezes is 91 x 10~* m3. (Given latent heat of ice = 3.36 x 105
t V Joule / Kg).
( (b)j) What is the significance of the thermodynamic potentials? [2]
v 5.(a) What is meant by degeneracjdf a given energy level?
(b) What are micro-states in a thermodynamic assembly?
(c) For an assembly of particles obeying B-E distribution the thermodynamic [4+1] probability is given by:
[1]
[1]
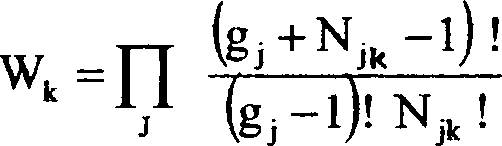
Obtain the B-E distribution function and show graphically, how the average
occupation number per state in any level varies with energy of the state.
, j. -
6.(a) Show that the principle of equipartition of energy follows from the Maxwell- [5+2] Boltzmann classical distribution function. Mention the limitations of this principle.
Also mention the cases where this principle is not applicable.
OR
Starting from the first principles, obtain the Plancks radiation formula for spectral [4+2+1] energy distribution of black body radiation. Show that for higher values of wavelengths this formula reduces to Rayleigh-Jeans law. What is ultraviolet catastrophe?
|
Attachment: |
| Earning: Approval pending. |
