Indian Institute of Technology Kharagpur (IIT-K) 2007 B.Tech Biotechnology Biochemical reaction engineering
|
Date of Examination................... End-Autumn Semester 2007-2008 .FN/AN Full Marks : 50 |
2nd yr.B.Tech. |
Subject No. BT21105 Subject: Biochemical Reaction Engineering & Bioenergetics No. of Students : 30 of the Department of Biotechnology
Instruction : Answer all questions from Group-A and any two questions from group-B. Answer to the point. Assume suitable data with necessary justification, if required.
2
2. a) In PS II which molecule is electron donor which molecules are electron acceptor. What is function of
|
Peso molecule on PS II? 1+1 |
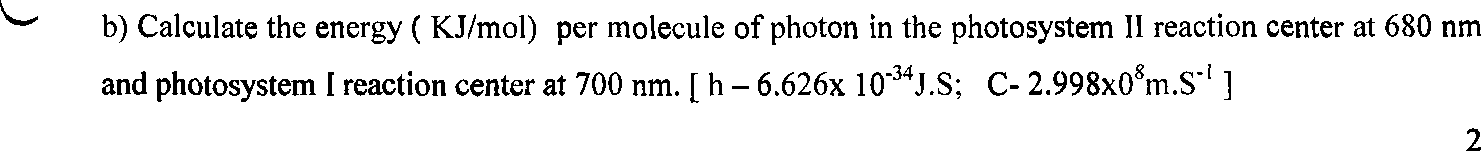 |
The release of one dioxygen molecule requires the transfer of 4 electrons from 2 molecules of water to 2 molecules of NADP+. Given the Emj of 2H2O 2H+ + O2 couple as +0.82 V and Em,7 of NADP++ 2e +2H+ NADPH couple as -0.32 V. Calculate the net thermodynamic efficiency of the electron transfer
steps of photosynthesis.
3
3. i) Enumerate the equations related to enthalpy, entropy and Gibbs free energy with heat capacity and temperature, ii) Define Tm of protein unfolding and how will you calculate fraction unfolded from a typical thermograph of protein unfolding due to increase of temperature. Iii) What is the significance of
the measurement of ACP of protein unfolding.
2 + 2+1
4. a)Binding constant ( K) of ligand( X) with macromolecule( M) is accurately determined by titration calorimeter. Enumerate the equation related to heat change ( dQ) with change in [MX} concentration, molar enthalpy and cell volume. 1
b) How the dimensionless parameter C is related with macromolecular concentraion and binding constant (K). What is the range of C by which binding constant ( K) could be determined by using titration calorimeter. 2
c)Describe in brief the thermodynamic signature optimization process for the selection of HIV protease inhibitor drug. 2
Group-B
5. a) An enzyme is immobilized on the surface of a non-porous solid matrix. Assuming that external
mass-transfer resistance for substrate is not negligible and that the Michaelis-Menten equation describes the intrinsic kinetics. Derive an expression which indicates the explicit form of the coefficients in a Lineweaver-Burk plot. Find out the values of vmax, Km and km(mass-transfer coefficient)?
5.5
b) Glucose is converted to fructose by using immobilized glucose isomerase. Find out the height of the immobilized enzyme column? Following data are given:
1. Diameter of the column = 5 cm.
2. Particle size 30/40 mesh (about 0.71 mm average diameter, dp),
3. Glucose concentration in feed at 60C = 500 g/1,
4. Glucose conversion efficiency = 60% w/w,
5. Feed viscosity = 3.6 c.p. at 60C,
6. Feed density = 1.23 g/ml at 60C,
7. Substrate diffusivity = 0.21 x 10'5 cm2/sec at 60C,
8. Void fraction = 0.35. 7
(a) What do you mean by DamkOhler number and effectiveness factor of an immobilized enzyme process? Find out the mathematical correlation between them. Explain the diffusion-limited regime of the immobilization process. 2+2+1.5
b) Write the Monod chemostat models. Derive the equation for finding out the cell mass and substrate concentration in a chemostat under steady state conditions. Write the mathematical expressions for the Dmax and DwaSh0ut. Explain the importance ofDwaShout for the operation of a CSTR system.
2+1+2+2
y, i) Candida utilis utilizes xylose at any concentration constitutively with a maximum growth rate of
0.31 h'1 and has a saturation constant (Ks) of 2.6 g/I and a cell yield on substrate (IVs) of 0.5 g/g. C. utilis can also metabolize galactose (with Ks = 0.8 g/1 and Yx/S = 0.5 g/g), but can only metabolize galactose at xylose concentrations at or below 0.5 g/1. If we have a feed stream that contains 10 g/1 of xylose and 30 g/1 of galactose entering a chemostat containing C. utilis. Find the following:
a. D (h'1) at which galactose begins to be metabolized.
a. Dilution rate for maximum cell productivity (Dopt)
b. Maximum ceil productivity
2+2+2
ii) D-(-)-4-hydroxyphenylglycin is the optically active intermediate in the synthesis of the broad-spectrum antibiotics amoxicilline. This intermediate is among others produced from a hydantoin derivative by means of the enzyme hydantoinase. The hydantoin derivative is poorly soluble in water, about I kg m'3. The price of the substrate is cost determining and degree of conversion should therefore be very high, at least 99%.
Calculate the volume needed to produce 100 kg of product per day by immobilized hydantoinase in a
- batch reactor 2.5
- CSTR 2
- Plug-flow reactor. 2
Data:
Michaelis-Menten reaction kinetics with vmax = 1.5 x 10-4 kg s'3 m'3 biocatalyst and Km = 5 x 10'
3 kg m3
Yp/s = 1 kg kg1 Degree of conversion 99%
Down-time for batch reactor 12 h
The activity of the biocatalyst can be assume to be constant in time
The reactor contain 0.1 m3 immobilized biocatalyst per m3, except the plug-flow reactor, which is packed with 0.5 m3 immobilized biocatalyst per m3 reactor.
|
Attachment: |
| Earning: Approval pending. |
