Indian Institute of Technology Kharagpur (IIT-K) 2008 B.Tech Electroanalysis and sensors - Question Paper
Vr-
U\
-L
L_ _ _
-V
INDIAN INSTITUTE OF TECHNOLOGY
Time: 2 Hrs. Full Marks: 50 No. of Students: 11
Sub.No. CY60019 Sub. Name: Electroanalysis and Sensors
f
1 __
>* ____
Nvi
'v>
Autumn Semester2008
Sr *yj''
Dept. Chemistry
*
" Answer all questions
Answer au questions f
1. Derive the expression for () current and (b) charge in potential step experiments using Ficks law of diffusion. t (5+5)
2. (a) Calculate the voltammetric peak current for the following reversible redox reaction in aqueous solution at the scan rate of 100 mV/s. The concentration and diffusion coefficient of the redox species are 0.5 mM and 6 x 10'6 cmV1; the experiment has been performed with a glassy carbon disk electrode of diameter 1.6
(3)
|
j> V o | 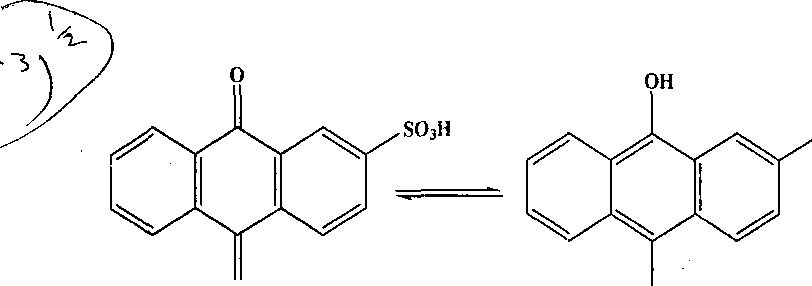 |
so3h |
) d*
|
,L) |
 |
0 OH
ratio of anodic-to-cathodic peak current (Ip7lpc) for this redox reaction increases while decreasing
v_ihe"scan rate- Give suitable reason for the observed increase in the ratio. (3)
3. The redox reaction of the co-factor PQQ confined on an electrode surface of 0.05 cm2 shows ideal voltammetric response with an anodic peak current of 10 jjA at the scan rate of 1000 mV/s. Calculate the surface coverage of PQQ. (4)
4. What are redox mediators? Explain the application of mediators in the development of biosensors.
(2+5)
5. Write down the structure of (a) redox unit in glucose oxidase and (b) the cofactor NADH. (2+2)
> / b) The rati
Consider the following reversible redox reaction involving one electron transfer in neutral solution:
0+1 e
R
In a chronocoulometric experiment, potential of the electrode (area: 0.15 cm2) was stepped from 0 to -0.7 V in an inert atmosphere. The total charge passed during the electrode reaction was 50 iC. The charge due to the double layer capacitance and the redox reaction of the species in solution were 10 and 25 C, respectively. Calculate the surface coverage of the adsorbed species. (6)
* *
7. Explain the mechanism for the electrocatalytic oxidation of hydrogen peroxide using the surface adsorbed macrocyclic nickel(II) complex. (7)
8. Explain with suitable example: (a) Reconstitution of redox enzymes for biosensing (b) electrical double
layer.
(6)
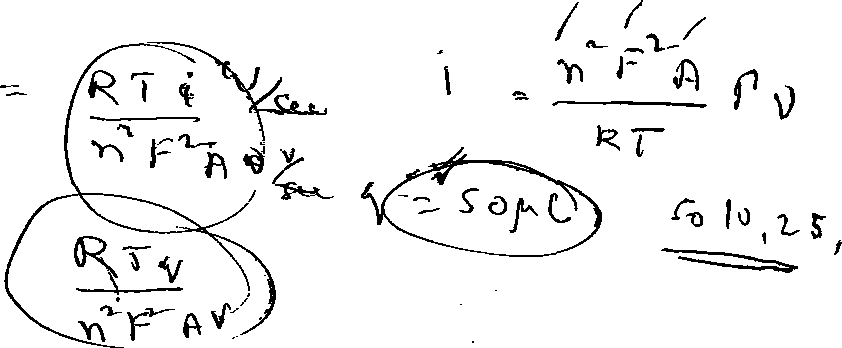
f
Cvr\
|
Attachment: |
| Earning: Approval pending. |
