Indian Institute of Technology Kharagpur (IIT-K) 2006 B.Tech Chemical Technology Reaction engineering - Question Paper
Department of Chemical Engineering End-Autumn Semester Examination, 2006
Name of the subject: Reaction Engineering No. of students: 34
Code of the subject: CH34009 Total no. of marks: 50.
PART-A
l.a) Considering irreversible reactions in parallel (both are elementary first order reactions) as
A- R and A- S having rate constants kl and k2 respectively,
how do you evaluate the rate constants? 3
. b) How do you illustrate the behaviour of a reaction if the order of the reaction shifts from low to high when reactant concentration falls? 3
2. a) The liquid phase reaction A + B- C + D takes place in a CSTR of volume
25 m3. The feed stream contains 5 kmol/m3 of A and 100 kmol/m3 of B. What volumetric flow rate and space-time is required to obtain 50% conversion of the limiting reactant? The reaction rate constant is 0.0001 m3/kmol.s. at the reaction temperature. 5
b) You have a choice to use plug or mixed flow reactor for a zero order reaction of
the type A - P + Q with a rate constant k. Which reactor would you choose
and why? 1
3. a) A first order liquid phase reaction is planned to carry out in two equal volume CSTRs. The reactors are connected in series and in parallel in two different cases. Compare the final conversion for two arrangements of the reactors.
Data: Fa0 = 10 Kmol/s, k= 1 s"1, Ca0 = 1 Kmol/m3, Vcstr= lm3 5
b) For a recycle reactor, if we go on increasing the recycle ratio what type of flow should we expect for a reactor? 1
4.a) Draw different types of contacting patterns in continuous flow reactors to give desired product distribution. 2
b) For the parallel decompositions of A, where R is desired,
R rR= 1
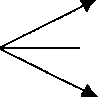 |
T rT = CA2 |
S rs = 2Ca with Ca<>= 1
What is the maximum Cr we may expect in isothermal operations
i) in a mixed reactor,
ii) in a plug flow reactor.
4
PART B
5. (a) for a non-isothermal spherical catalyst pellet, show that the energy balance can be written as
where ke is the effective thermal conductivity. Write the boundary conditions of the above equation. Derive an expression for the maximum temperature rise in a nonisothermal spherical pellet.
(b) The catalytic cracking of gas oiI(Average Mol wt.=120) was carried out at 500C and 1.2 bar pressure with a spherical catalyst particles of 0.33 cm. diameter. The mean pore radius of the catalyst is 30* 10'8 cm, having a pore volume of 0.35 cm3/gm, at the given pressure. The diffusion is Knudsen type. The rate constant was 0.25 cm3/gm.s. Assume a tortuosity factor of 3.0, Calculate the effectiveness factor of the catalyst and make a comment on the effect of internal diffusion. [5+4]
6. In a laboratory RTD- experiment injecting a pulse input in an isothermal tubular reactor the following data were obtained.
|
t, min |
0 |
10 |
30 |
40 |
50 |
60 |
90 |
100 |
|
C, mg/1 |
0 |
1.8 |
5 |
5.5 |
5.2 |
4.2 |
1.8 |
0 |
|
Attachment: |
| Earning: Approval pending. |
