Thapar University 2006 B.Tech Biotechnology Biochemistry - Question Paper
Thapar Institute Of Engineering And Technology :Patiala
BT-006
Biochemistry
End Semester exam
Thapar Institute of Engineering and Technology End Semester Examination t/l BTech. Biotechnology
' BT006 Biochemistry 10.12.06
Time: 3hour Max. Marks: 36
Note: Attempt any Five questions.
All questions carry equal marks. All parts of a question are to be attempted in continuation_
1. (i) The electron transport chain consists of four structured complexes of functionally 5.2
related electron carriers. Summarize the components of the complexes and their electron transfer reactions.
(ii) In electron transfer, only the quinine portion of ubiquinone undergoes redox; the 2 isoprenoid side chain remains unchanged. What is the function of this chain?
2. (i) What is p oxidation? How many turns of the fatty acid oxidation cycle are required 3
to oxidize arachidic acid (a 20 C fatty acid) completely to acetyl CoA.
(iii) Show the complete oxidation of palmitic acid represented by an overall equation.
(iii) Palmitic acid is activated in the cell by conversion to its RCO-S-CoA derivative. 2 Given that standard free energy of hydrolysis is -7.5kcaI/mol for RCO-S-CoA and -1.1 kcal/mol for p -phosphate linkage of ATP. Calculate A G and the equilibrium 2.2 constant for the activation of palmitic acid RCOOH
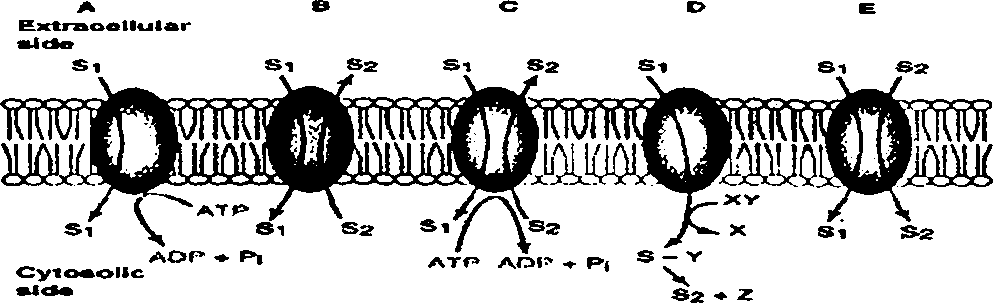
3. Discuss different types of transport (A - E) across the membrane with suitable examples 7.2 as shown in the representation given below
4. (i) Show the condensation reaction of acetyl CoA and the decarboxylation reaction 3
catalyzed by isocitrate dehydrogenase in TCA cycle.
(ii) Although molecular oxygen does not participate directly in the citric acid cycle, the 2 cycle operates only when oxygen is present. Why?
(iii) Describe the function and name the enzyme (s) in the following two cases below
(a) Enzyme that catalyzes ATP synthesis in glycolysis. 2.2
(b)Enzymes that catalyze the irreversible reactions of glycolysis.
5. (i) What assumptions are used in deriving the Michaelis-Menten equation? 2
(ii) The kinetics of an enzyme is measured as a function of substrate concentration in 3.2 the presence of inhibitors and in their absence. Explain the correlation between the
Km and Vmax in the presence and absence of these inhibitors.
(iii) Show how Km and Vmax values are estimated using the Lineweaver-Burk plot. 2
6. (i) Explain the structure and functions of Biomolecules. 4
(ii) Name the disaccharide shown below; What are its constituent sugars? Distinguish 3.2
between anomers and epimers with examples.
|
uoAr~ |
ft ft V 1 |
T TA |
|
H)1 | ||
|
- * | ||
|
H |
OH |
V* |
|
Attachment: |
| Earning: Approval pending. |
