Indian Institute of Technology Guwahati (IIT-G) 2007 JAM Chemistry - Question Paper
JAM 2007 Chemistry
Full ques. Paper in attachment
Chemistry Paper 2007
IMPORTANT NOTES FOR CANDIDATES
Attempt ALL the 44 questions.
Questions 1-30 (objective questions) carry three marks each and questions 31-44 (subjective questions) carry fifteen marks each.
Write the answers to the objective questions in the Answer Table for Objective Questions provided on page 13 only.
1. The compound, which
(i) reacts rapidly with acetyl chloride,
(ii) does not react with 2,4-dinitrophenylhydrazine and
(iii) does not form a yellow precipitate with excess of iodine in aqueous alkali is
(A) acetone
(B) diethyl ether
(C) 2-methyl-2-propanol
(D) ethanol
H2OH -OH -Cl
CHO
H-
H-
-OH -Cl CH2OH 1
H-
H-
CHO
2
The given compounds 1 and 2 are
(A) identical
(B) diastereomeric
(C) enantiomeric
(D) constitutionally isomeric
3. The correct order of dipole moments (//) of the following compounds is
1. CH3CH2CH2CHO 2. CH3CH=CHCHO 3. CH3CH2CH=CH2
(A) JU1>jU2>M3
(B) H2>JU3>Mi
(C) Mz >Mi>M2
(D) ju2>ju1>ju 3
4. Which one of the following compounds gives positive test for both nitrogen and halogen with its Lassaignes extract?
(A) CH3NH2.HCI
(B) NH2OH.HCI
(D) H2NNH2.HCI
5. Which one of the following compounds is optically active?
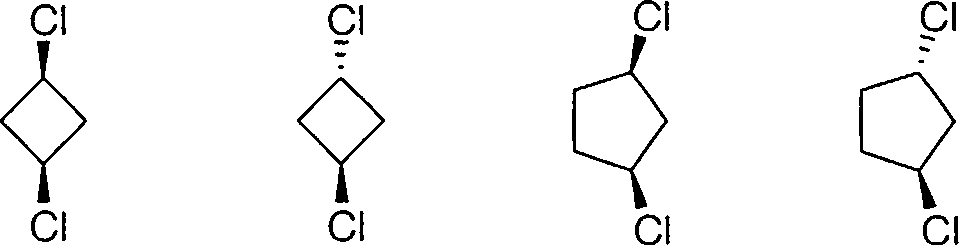
(1) (2) (3) (4)
|
(A) |
1 |
|
(B) |
2 |
|
(C) |
3 |
|
(D) |
4 |
6. The compounds that react with aqueous NaHCC>3 to release CO2 are
O
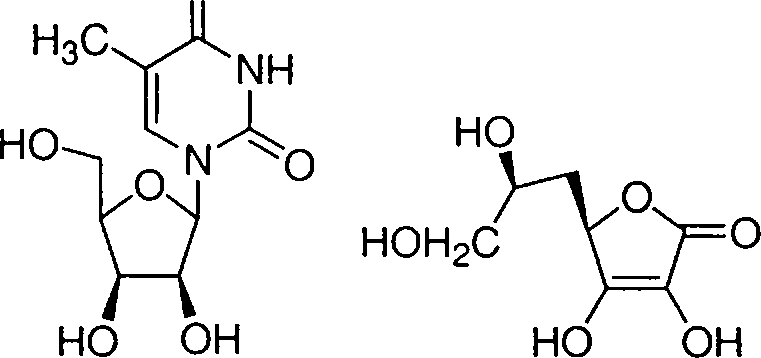 |
|
(3) (4) |
. HO O (2)
(A) 1 and 3
(B) 2 and 4
(C) 2 and 3
(D) 1 and 4
7. The complementary strand of DNA for the following single stranded DNA sequence, 5' -A-T-C-A-T-G-C- 3' is
(A) 5'-A-T-C-A-T-G-C-3'
(B) 5' -T-A-G-T-A-C-G- 3'
(C) 5' -G-C-A-T-G-A-T- 3'
(D) 5' -C-G-T-A-C-T-A- 3'
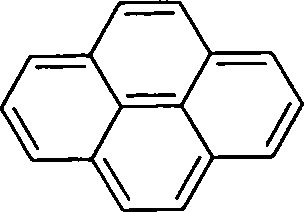
8. The value of n for the following molecule according to Hiickels rule is
(A) 16
(B) 4
(C) 3
(D) 14
H
OH
nh2
OH
(B)
NH2
OH

(C)
NH2
(D)
OH
NH2
10. The main product obtained in the following reaction is
O O
1. MeMgBr/dry ether
[?]
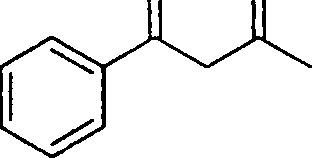
2. H7 HoO
|
O O |
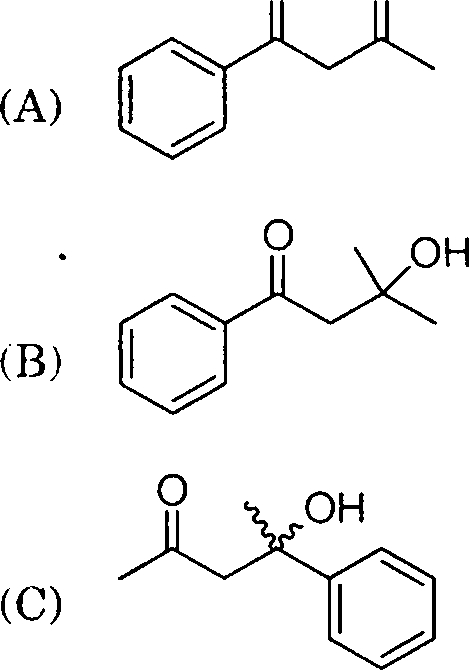 |
O O
(D)
11. For a reaction with rate equation -dC/dt = kC2, Co and C are the concentrations of the reactant at time 0 and t respectively. If 10 minutes were required for Co to become Co/2, the time required for Co to become Co/4 is
(A) 10 min
(B) 20 min
(C) 30 min
(D) 40 min
12. For a cyclic process performed by an ideal gas, changes in some thermodynamic functions are zero. Indicate the set in which all the functions are zero.
(A) w, AE, AH, AG
(B) q, AS, AH, AA
(C) q, AE, AS, AG
(D) AE, AS, AH, AA
13. The plot of Gibbs free energy G and the extent of a reaction is given below for the reaction A B. If JIa and p.B are the chemical potentials of A and B respectively, the
INCORRECT statement is
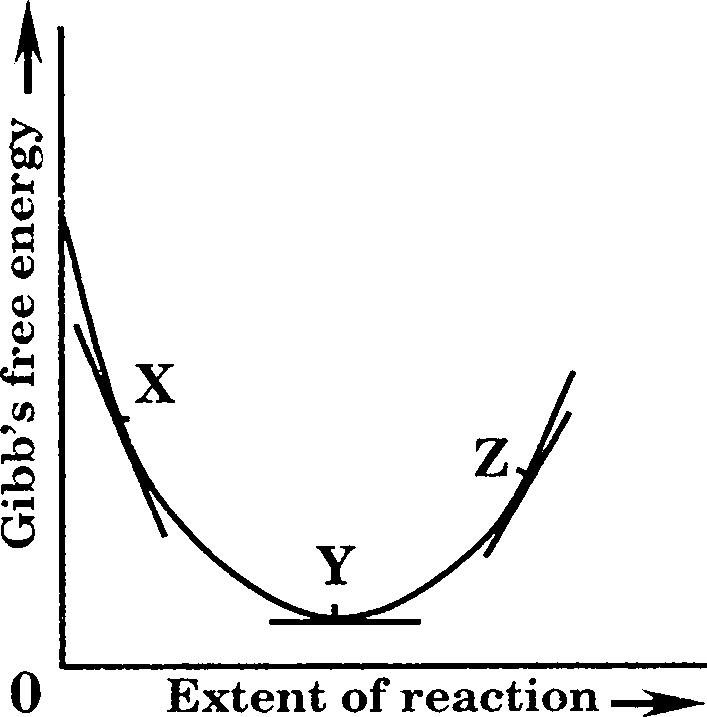
(A) at point X, ]JlA > PB
(B) at point Y, AG = 0
(C) at point Z, Pa > HB
(D) at equilibrium, the composition of the reaction mixture can be identified
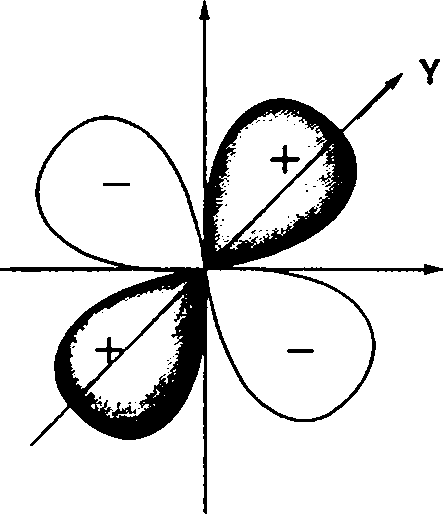
(A) positive
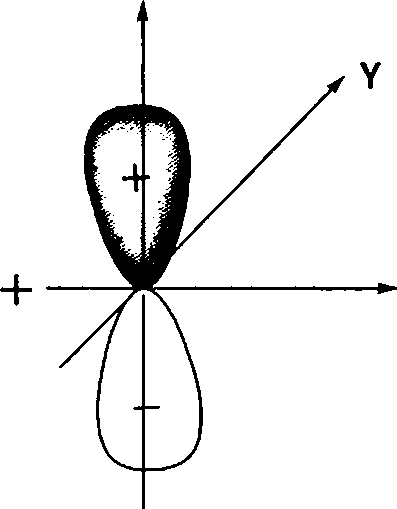
(B) negative
(C) zero
(D) no overlap
15. The pH of a 1.0 x 103 M solution of a weak acid HA is 4.0. The acid dissociation constant Ka is
(A) 1.0 xlO"3
(B) l.OxlO4
(C) 1.0 xlO-5
(D) 2.0 xlO-5
16. The normalisation constant A for the wavefunction where 0 < <p < In is
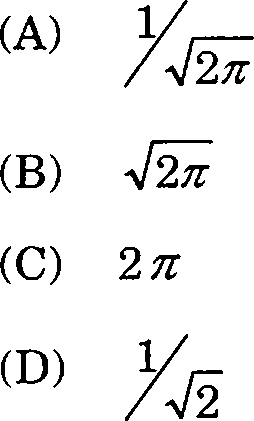
17. The standard potential of a Daniel cell is + 1.10 V and the equilibrium constant for the cell reaction is 1.5 x 1037. It can be concluded that
(A) zinc oxidises copper
(B) displacement of copper by zinc goes to near completion
(C) copper oxidises zinc
(D) displacement of zinc by copper goes to completion
18. Which one of the following figures, showing kinetic energy of the ejected electron versus the frequency (y) of the incident photon, represents the Einsteins photoelectric effect?
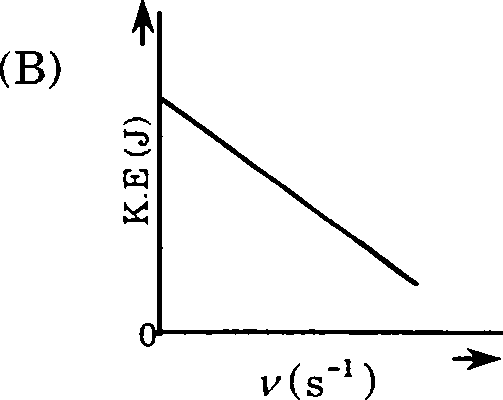
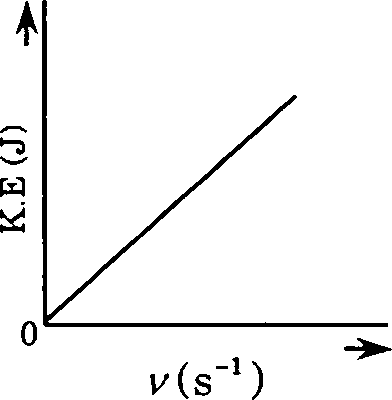
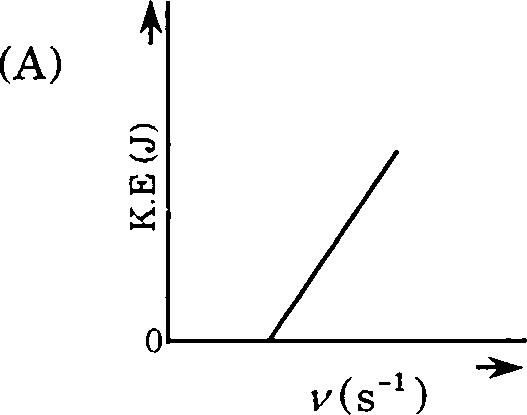
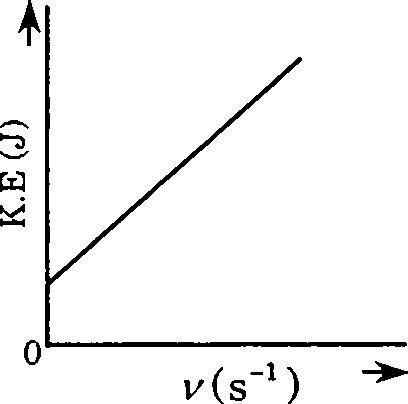
19. An aqueous solution containing 0.01 M FeCb and 0.06 M HCIO4 has the same ionic strength as a solution of
(A) 0.09 M NaCl
(B) 0.04 M Na2S04
(C) 0.06 M CuSC>4
(D) 0.03 M H3PO4
|
(A) |
H20 |
|
(B) |
O2" |
|
(C) |
O; |
|
(D) |
of |
21. The solid-liquid phase diagram for the Mg-Zn system is shown in the figure below where the vertical line at X(Mg) = 0.33 represents the formation of a congruent melting compound MgZn2. The figure is divided into seven regions depending upon the physical state of the system. The composition of the region #6 represents
700 600 500
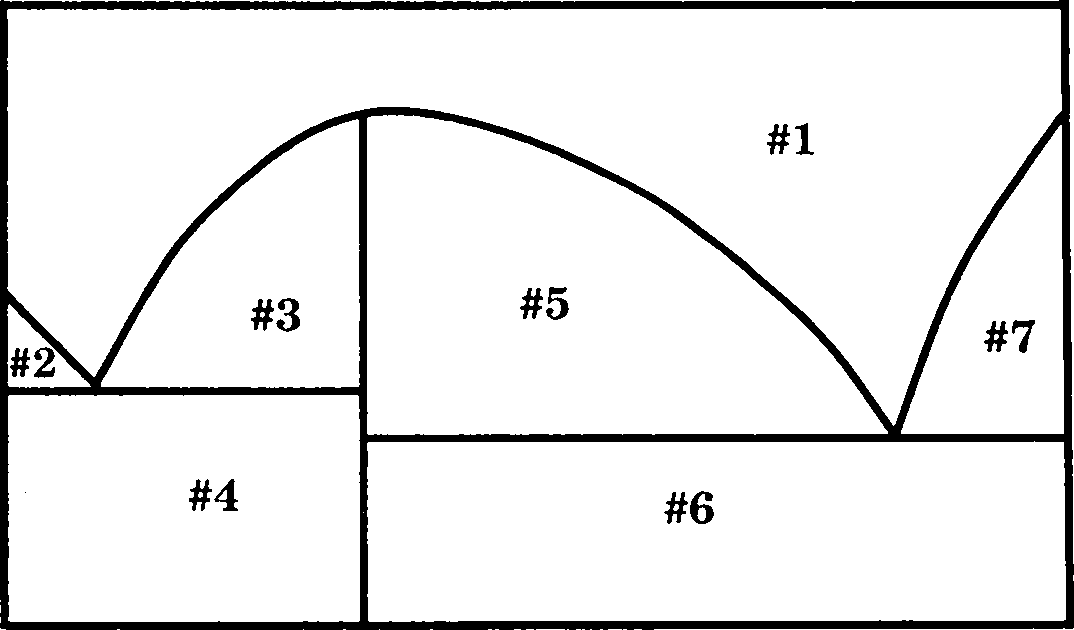
O
5 400
300 200
0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Zn x(Mg) Mg
(A) single phase of a solution of Mg and Zn
(B) two phase region between the solid Zn and solid MgZm
(C) two phase region between the liquid and solid MgZm
(D) two phase region between solid Mg and solid MgZn2
22. In the extraction of metals from their ores, which one of the following reduction methods can bring about a non-spontaneous reduction?
(A) electrolytic reduction
(B) reduction by carbon
(C) reduction by another metal
(D) reduction by hydrogen
23. The correct order of the ionic radii is
(A) In3+ > Sn4+ > Sr2+ > Rb+
(B) Sn4+ > In3+ > Sr2+ > Rb+
(C) Rb+ > In3+ > Sr2+ > Sn4+
(D) Rb+ > Sr2+ >In3+ > Sn4+
24. The correct valence shell electronic configuration of the element with atomic number 22 is
(A) [Ar]4s23d2
(B) [Ar]3d4
(C) [Ar]3d24s2
(D) [Ar]4s24p2
25. The ligand with only sigma (o) bonding character is
(A) CN-
(B) CHg
(C) CO
(D) NO
26. Which one of the following species is NOT isoelectronic with CO ?
(A) N2
(B) CN"
(C) NO+
(D) 0+
27. During Wittig reaction, a phosphorus ylide gets converted to
(A) R3P
(B) R3P=0
(C) R3P+HOH
(D) R2PPR2
28. Which of the following reactions does NOT give H3PO4?
(A) Ca3(PC>4)2 + H2SO4-
(B) P406 + H2O -
(C) PCls + H2O -
(D) P4S10 + H2O -
29. The ionic radii of Ca2+ and F" are 100 pm and 133 pm respectively. The coordination number of Ca2+ in the ionic solid will be
(A) 8
(B) 6
(C) 4
(D) 2
30. The shape of CH3 ion is
(A) trigonal planar
(B) tetrahedral
(C) trigonal pyramidal
(D) linear
l.NaOH 2. H+
[R] CuH1202
1.CH:,MgBr (excess)
2. HBr
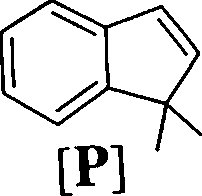
1.KMn04
2. NaI04
[Q] CuH1202
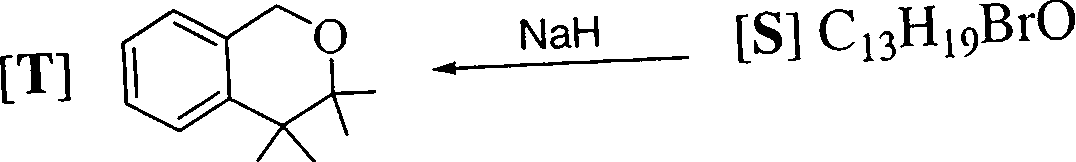
Identify the structures of the intermediate compounds Q, B and S. Show the transformai
for each step.
For the following scheme of transformations, draw the structures of A, B, C and D. CHO
-OH -H -OH -OH CH2OH
H-
HO-
H-
H-
phenylhydra/.ine (3 eq) benzaldehyde (excess)
[B
H+
acid catalysed rearrangement
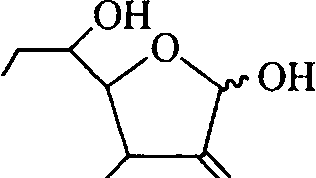 |
|
HO O |
(D1 Br ,0] HI0 H0
(9)
(b) Complete hydrolysis of a pentapeptide with 6 N HC1 at 110 C in a sealed tube gave
2 equivalents of glycine, one equivalent each of tyrosine, leucine and phenylalanine. Reaction of the pentapeptide with Sangers reagent (2,4-dinitrofluorobenzene, DNFB) and subsequent hydrolysis gave the DNFB derivative of tyrosine. Chymotrypsin cleavage of this peptide yielded tyrosine, leucine and a tripeptide. Deduce the sequence of the pentapeptide. (6)
33. Complete the following reactions with appropriate structures for E, F, G, H and I.
O
NaN02 / HCI r _ n Zn / AcOH COOEt --- [ E ] -- [ F ]
(a)
heat
[F]
[G]
O'
-C02Et
(9)
alkaline KMn04 r Tr , heat, acetophenone
(b)
(6)
34. (a) Account for the following transformation with an appropriate mechanism. Give the structure of the Hofmann exhaustive mthylation product of 1,2-dihydro derivative of [X].
OH
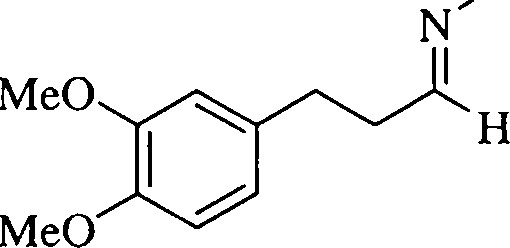
PCI,
MeO
(9)
XXi
[X]
MeO
(b) The optically pure ester [J] is hydrolysed in aqueous acetic acid to form a racemic mixture of cis-4,4-dimethyl-2-acetoxycyclopentanol [K]. Give a mechanistic explanation to account for the formation of [K] and the observed change in the optical activity.
[K]
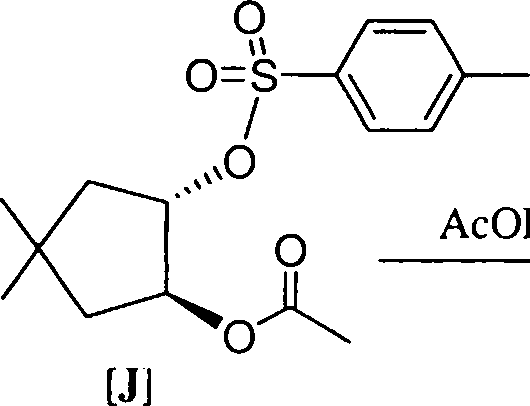
AcOH/ H20
35. (a) M is a first row transition metal. MCb on treatment with aqueous ammonia gives a blue colored solution of complex N. A solution of MCb also gives a bright red precipitate of complex O with ethanolic dimethylglyoxime.
(i) Identify M and draw the structure of O.
(ii) Determine the hybridisation of M in complex N.
(iii) Identify the paramagnetic complex. (9)
(b) [Cr(H20)G]3+ gave an absorption at 208 kJ/mol which corresponds to Ao. Calculate the crystal field stabilisation energy of this complex in kJ/mol. (6)
36. (a) Consider the ethers HsSiOSiHh and H3COCH3.
(i) Which ether has more Lewis base character?
(ii) Which angle [Si-O-Si and C-O-C] is greater?
Justify your answer.
(9)
(b) Starting from SiC>2, show how the following polymer is prepared industrially?
n
37. (a) A solution of metal ion (M2+) when treated with H2S gas gives a black precipitate A.
Precipitate A dissolves in hot concentrated nitric acid to give B along with elemental sulfur. The metal ion solution also gives a white precipitate C with an excess of KI. Write the chemical formulae of A, B and C. (9)
(b) Why are potassium permanganate solutions unstable in the presence of Mn2+ ions? In the quantitative estimation of iron present in iron ores dissolved in dilute HC1, titrations with dichromate are preferred over titrations with permanganate. Rationalise. (6)
38. (a) A12C16 and Al2Me6 are dimeric in gas phase. Draw their structures. Which compound has more Lewis acid character? Explain. (9)
(b) Arrange the halides SnCl2, PbCl2, SiCl2 in increasing order of their stability. Give reasons for your answer. (6)
39. (a) Acidification of an aqueous solution of yellow sodium chromate gives an orange colored compound A. A saturated solution of A on treatment with concentrated H2SO4 gives a bright orange solid B. Compound A in the presence of concentrated H2SO4 reacts with anion C to give a deep red colored liquid. Identify A, B and C. (9)
(b) 2g4 Po undergoes an a emission to give element X followed by a /? emission to give element Y.
(i) Write the valence shell electronic configuration of Y.
(ii) Indicate the groups of the periodic table to which X and Y belong.
(6)
40. (a) When an ideal monoatomic gas is expanded from 1.5 bar, 24.8 L and 298 K into an evacuated container, the final volume becomes 49.6 L. Calculate AH, AS and AG for the process. (9)
(b) The Maxwell distribution function for the distribution of speeds of molecules in gaseous systems is given as,
/<c)=44r)exp(-%J
Show that the most probable speed, cmps = (6)
41. (a) At 600 K and 200 bar, a 1:3 (molar ratio) mixture of A2 and B2 react to form an equilibrium mixture containing *ABg = 0.60. Assuming ideal gas behaviour, calculate Kp for the reaction
A2(g) + 3B2(g)F 2AB3 (g)
(9)
(b) A 50 mL 0.05 M solution of Fe(II) is titrated with 0.05 M solution of Ce(IV) in the presence of dilute H2SO4 at 25 C. Calculate the equivalence point potential and the equilibrium constant K in terms of log K.
[V+M2+) =+0.75 V, V+/ce3+) = +1.45V]
(6)
42. (a) The vapour pressure of D2O at 20 C is 745 mm Hg. When 15 g of a non-volatile compound is dissolved in 200 g of D2O, the pressure changes to 730 mm Hg. Assuming the applicability of Raoults law, calculate the molecular weight of the compound. (9)
(b) An enzyme following Michaelis-Menten kinetics was found to have highest activity at 37 C and pH 7.0. If the maximum velocity Vmax for this enzyme was 2.4 x 10"1 mol L_1s_1 with an initial enzyme concentration [E]0 = 2.4 nM, calculate the turnover frequency. (6)
43. (a) Consider the 4 n electrons in cyclobutadiene to be free particles in a 2-dimensional square box of length 2 A. Calculate the wavelength of the electronic transition from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). Also write down the normalised wavefunctions for the occupied degenerate states. (9)
(b) The reaction
is first order in both directions. At 25C, the equilibrium constant (K) of this reaction is 0.40. If 0.115 mol.dm-3 of cis-isomer is allowed to equilibrate, calculate the equilibrium concentration of each isomer. (6)
44. (a) With i, j and k as the unit vectors along X, Y and Z axes, express the vector PtP2 in the given figure in terms of the coordinates of Pi and P2. Also determine the dot products of the unit vectors i, j, k.
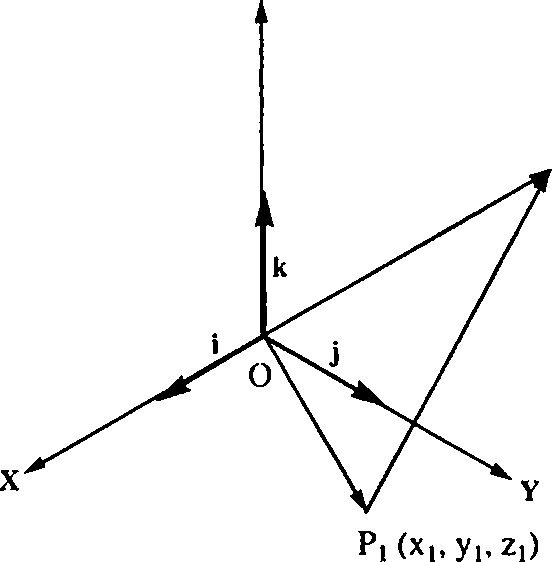 |
P2 (x2, y2> z2) |
(9)
(b) Deduce whether the matrices A and B commute or not.
|
'2 |
B = |
(1 |
i\ | ||
|
,0 |
1, |
,0 |
b |
(6)
|
Attachment: |
| Earning: Approval pending. |
