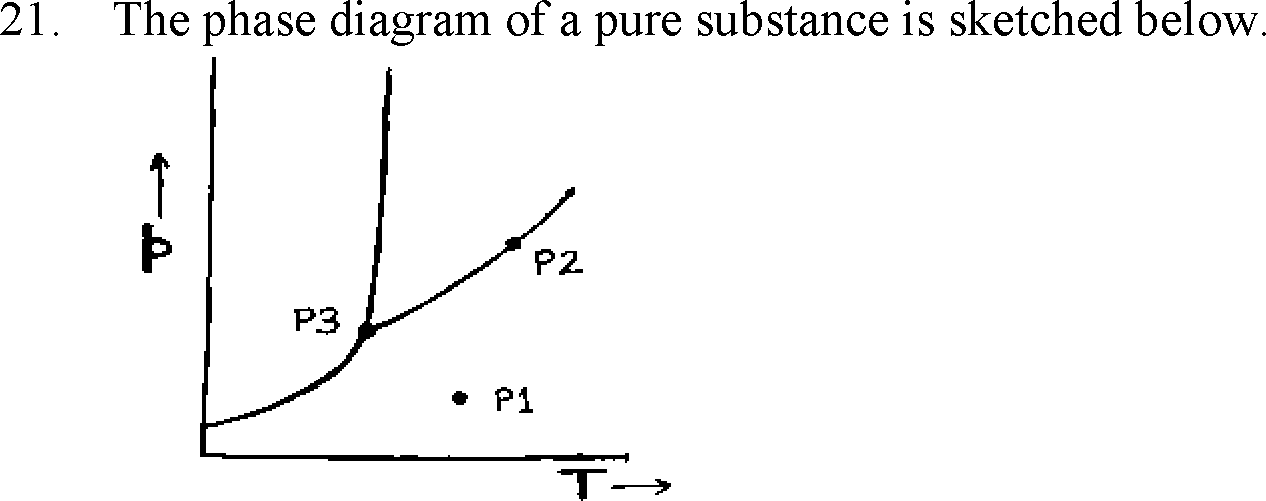Indian Institute of Technology Guwahati (IIT-G) 2006 JAM Chemistry - Question Paper
Wednesday, 23 January 2013 07:10Web
Page 1 of 2
JAM 2006 Chemistry
Full ques. Paper in attachment
JAM 2006
CHEMISTRY TEST PAPER
Useful Data
R T
2.303-= 0.059 V at 298.15 K
F
R = 8.3145 J.mol-1 K-1 F = 96500 C.mol-1 KW = 10-14 at 298.15 K me = 9 x10-31kg Kf = 1.8 C/ m for water h = 6.6 x10-34 J.s ln 2 = 0.69
Atomic number of Ti = 22, Cr = 24, Co = 27
NOTE: Attempt ALL the 44 questions. Questions 1- 30 (objective questions) carry three marks each and questions 31- 44 (subjective questions) carry fifteen marks each. Write the answers to the objective questions in the Answer Table for Objective Questions provided on page 13 only.
1. After the following interchanges of groups in the Fischer projection of 2-bromobutane, the configuration of (X) and (Y) will be
ch3
(A) X = R; Y = S (b) X = S; Y = R
(X)
(C) X = R; Y = R
(D) X = S; Y = S
|
CHO |
 |
|
CHO |
 |
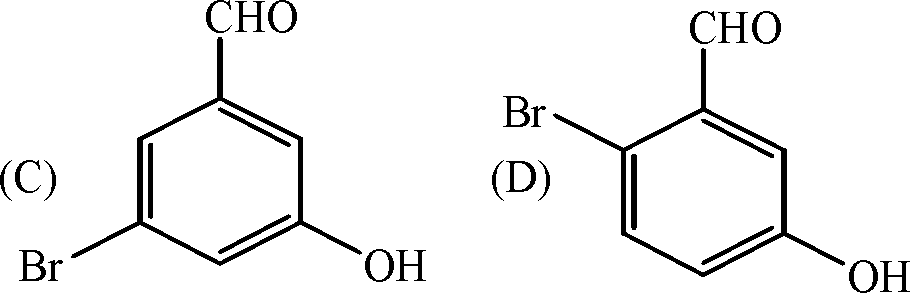
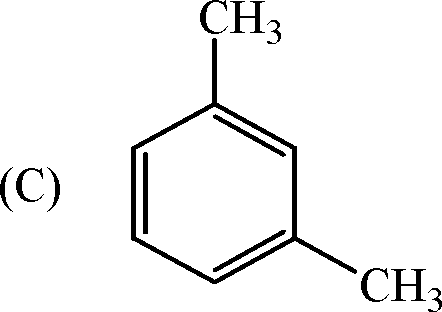
3. In the reaction sequence
the product (Y) is
(A) CH 3COCH 2COOCH 3
(B) CH3COCHCOOH
I
CH 3
4. The major product (X) in the reaction
(C) CH3COCH(CH 3)2
(D) CH 3COC 2H5
 |
3 AlCl |
C6H6 + CO + HCl
heat
gives positive test with Fehlings solution. The product is
(A) C6H5OH (B) C6H4(Cl)CHO (C) C6H4(OH)CHO (D) C6H5CHO
6. The compound (X) in the reaction sequence
CH 2COONa P2S3 Raney Ni
| -(X) - CH 3CH 2CH 2CH 3
CH 2COONa heat
o
(A) (i \ (B) | (C)
'S CH 2COOH
7. The major product of the reaction
OnO
|
OH OH
O |
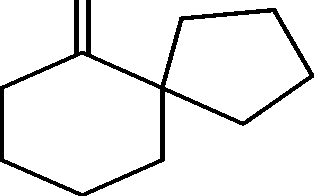 |
|
(C) |  |
|
\_/ |  |
0-3 (D)
OH
8. The increasing order of the acidity of the hydrogen marked in bold italics among the following is
 |
|
HH
II |
 |
|
H H I |
(C) II < I < III
(B) I < II < III (D) II < III < I
9. The major product of the reaction
HBr
|
(A) |  |
|
Br |
 |
10. The number of enantiomers of camphor
 |
|
O |
is
11. The decreasing order of the first ionization energy of the following elements is
(A) He > H > Be > B
(B) Be > B > H > He
(C) H > He > Be > B
(D) B > Be > He > H
12. If the values of Madelung constants of the following compounds are equal, then their lattice energy values decrease in the order
(A) KCl > NaF > CaO > AhOs
(B) Al2Os > CaO > NaF > KCl
(C) NaF > KCl > CaO > AhOs
(d) Al2Os > CaO > KCl > NaF
13. The fluoride, whose value of dipole moment is NOT equal to zero, is
(A) XeF4
(B) CF4
(C) SF4
(D) PF5
14. The decreasing order of the ionic nature of the following compounds is
(A) Lil > NaBr > KCl > CsF
(B) Lil > KCl > NaBr > CsF
(C) CsF > NaBr > KCl > Lil
(D) CsF > KCl > NaBr > Lil
15. The atomicity and the total number of bonds in the elemental white phosphorus molecule are, respectively,
(A) 4 and 6
(B) 6 and 4
(C) 4 and 4
(D) 6 and 6
16. The octahedral crystal field splitting (Ao) of d orbital energies of the following metal ions decreases in the order
|
(A) |
Co2+ |
> |
Co3+ |
> Rh3+ |
|
(B) |
Rh3+ |
> |
Co3+ |
> Co2+ |
|
(C) |
Rh3+ |
> |
Co2+ |
> Co3+ |
|
(D) |
Co3+ |
> |
Co2+ |
> Rh3+ |
17. The half-life of a radioactive nuclide is 20 years. If a sample of this nuclide has an activity of 6400 disintegrations per minute (dis/min) today, its activity (dis/min) after 100 years would be
(A) 850
(B) 1600
(C) 200
(D) 400
18. The average value of C-C bond order in graphite is
(A) 1
(B) 3/2
(C) 3/4
(D) 4/3
19. The optical absorption spectrum of [Ti(H2O)6]3+ has its absorption maximum at 20300 cm-1. The magnitude of crystal field stabilization energy in cm-1 is
(A) 8120
(B) 16240
(C) 24360
(D) 50750
20. In inorganic qualitative analysis, H2S in acidic medium will NOT precipitate
(A) HgS
(B) ZnS
(C) CuS
|
(D) CdS |
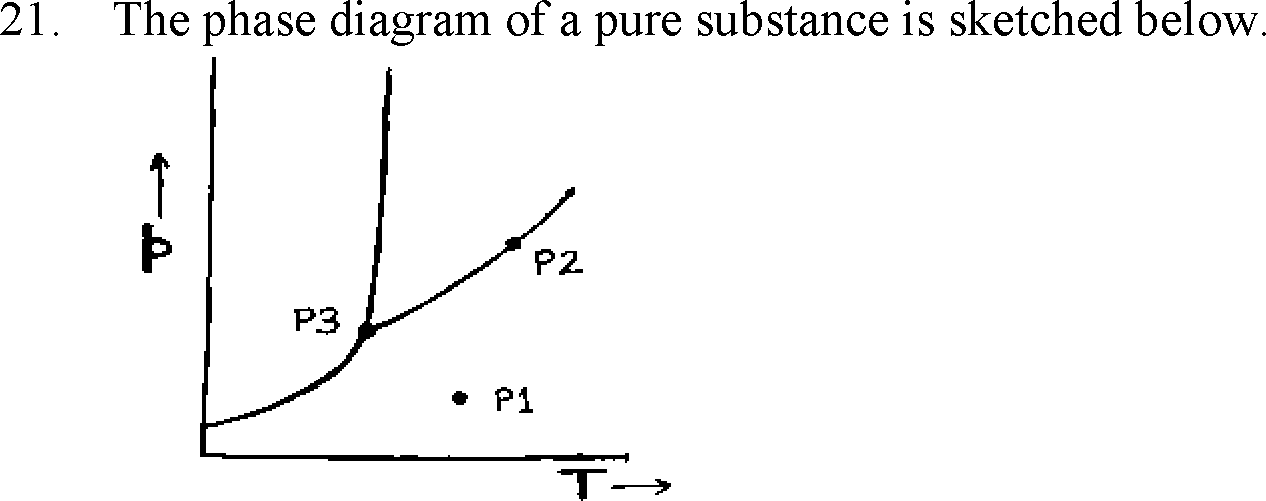 |
The number of degrees of freedom at points P1, P2 and P3, respectively, are
(A) 2, 1, 0
(B) 1, 2, 0
(C) 2, 0, 1
(D) 0, 2, 1
The solubility products (Ksp) for three salts MX, MY2 and MZ3 are 1x10-8, 4x10-9 and 27x10-8, respectively. The solubilities of these salts follow the order
(A) MX > MY2 > MZ3
(B) MZ3 > MY2 > MX
(C) MZ3 > MX > MY2
(D) MY2 > MX > MZ3
23. The temperature (T) dependence of the equilibrium constant (K) of a chemical reaction is correctly described by the following statement:
(A) For an endothermic reaction, the slope of ln K vs 1/T plot is positive
(B) For an exothermic reaction, K is proportional to T
(C) For an exothermic reaction, K at a higher temperature is lower than K at a lower temperature
(D) If AH is independent of temperature, the change in K with T is smaller at lower temperatures
24. When the concentration of K+ across a cell membrane drops from 0.01 M to 0.001 M, the potential difference across the membrane is
(A) 0.0 V
(b) 0.0059 V
(c) 0.059 V
(D) 0.59 V
25. The statement that is correct for both electrochemical (galvanic) cells and electrolytic cells is
(A) AG = - nFE
(B) Free energy decreases in both cells
(C) The cell potentials are temperature independent
(D) Chemical energy is converted into electrical energy in both cells
26. The molar heat capacity at constant volume of a colourless gas is found to be 25 J.mol-1K-1 at room temperature. The gas must be
(A) N2
(B) O2
(C) CO2
(D) SO2
27. The wavefunction for a particle (moving in a ring) is (2n)-12 exp(2i), where is the polar angle. The probability of finding the particle in a small interval d when the value of = n/2 is
(A) d
(B) d / 2n
(C) d exp (in)
(D) d exp (in) / 2n
28. An electric current of 0.965 ampere is passed for 2000 seconds through a solution containing [Cu(CH3CN)4]+ and metallic copper is deposited at the cathode. The amount of Cu deposited is
(A) 0.005 mol
(B) 0.01 mol
(c) 0.02 mol
(D) 0.04 mol
29. The Maxwell-Boltzmann distribution for molecular speeds is shown in the following figure.
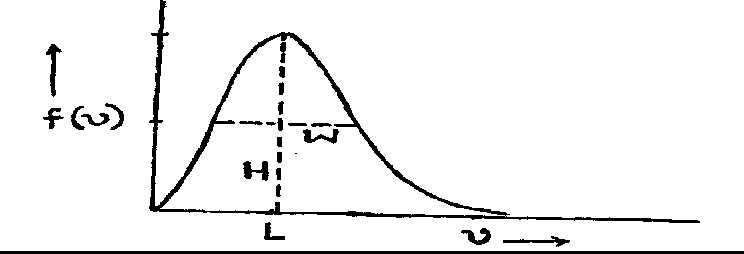
In the figure, H is the height of the peak, L is the location of the maximum and W is the width at half height. As the temperature is decreased,
(A) H increases, L decreases and W increases
(B) H increases, L decreases and W decreases
(C) H decreases, L decreases and W increases
(D) H decreases, L decreases and W decreases
30. A system undergoes two cyclic processes 1 and 2. Process 1 is reversible and process 2 is irreversible. The correct statement relating to the two processes is
(A) AS (for process 1) = 0, while AS (for process 2) 0
(B) qcyclic = 0 for process 1 and qcyclic 0 for process 2
(C) More heat can be converted to work in process 1 than in process 2
(D) More work can be converted to heat in process 1 than in process 2
31. Identify reagent (P) and write the structure of products (Q, R, S and T) in the following series of reactions (15)
|
NH' |
 |
i) HNO3/H2SO4, 20 oC
ii) H/H2O, heat
H5C6
(dextro)
i) what is the optical activity of the product?
ii) draw the energy profile for the reaction
iii) write the structure of the intermediate
iv) what is the effect of doubling the concentration of KOH on the rate of the reaction?
v) if aqueous KOH is replaced by alcoholic KOH, write the structure of the product formed.
(15)
33. (a) Suggest a method for the following transformation involving minimum number of steps.
|
CH |
 |
|
CH |
 |
Indicate the reagents/reaction conditions required at each step clearly.
(b) A dipeptide on hydrolysis gives two amino acids (X) and (Y). If the dipeptide is first treated with HNO2 and then hydrolysis is carried out, (X) and lactic acid are obtained. (X) on heating gives 2,5-diketopiperazine as shown below. Identify (X) and (Y), and write their sequence in the dipeptide. (9)
.O
34. Identify the compounds A, B, C, D and E in the following reactions
O
I) OH", PhCHO
3 -[ B ]
II) H+, heat
11) Zn / H 2O
35. (a) Why do the bo1l1ng po1nts of the followmg compounds vary 1n the order,
H2O > H2Se > H2S ? (6)
(b) Identify the products 1n the reaction of CCl4 and SCl4 w1th water. Justify your answer.
(9)
36. (a) Write the steps involved 1n the production of pure elemental sticon from slca. (9)
(b) Both the products A and B, 1n the followmg reactions, conta1n boron and mtrogen. Ident1fy A and B. (6)
C6H5Cl L1AlH 3 NH4Cl + 3 BCl3 - A - B
heat THF
37. (a) Addition of potassum oxalate solution to a hot solution of potass1um dchromate
contanng dlute sulfuric add leads to effervescence and formation of potassum tr1soxalatochromate(III). (1) Wr1te the chem1cal formula of the chrom1um complex formed. (11) Write the balanced chemical equation for the formation of the complex. (111) Calculate the room temperature sp1n"only magnet1c moment, 1n Bohr magnetons, of the complex. (9)
(b) Write the structures of poss1ble 1somers of [CoCl2(en)2]Cl (6)
38. (a) Draw the un1t cell of CsCl lattice. Draw the (100) and (110) planes separately and
1nd1cate the pos1t1ons of ces1um and chloride 1ons. (9)
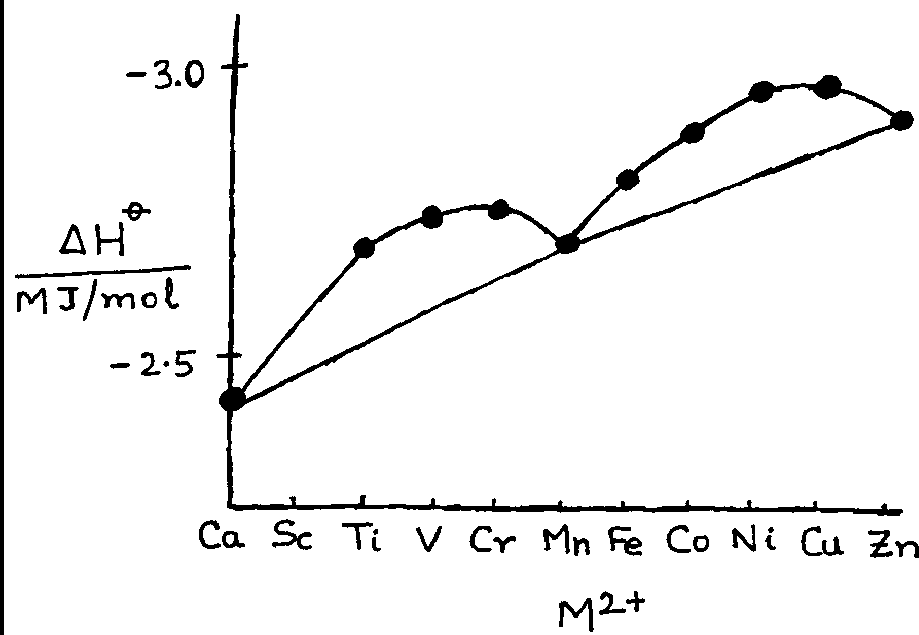
(b) The hydration enthalpies of divalent metal ions of ten elements from calcium to zinc are plotted against their atomic numbers. Why do the hydration enthalpies of only three elements, Ca, Mn and Zn fall on a straight line, whereas values for other metal ions deviate from this line? (6)
39. (a) 5 grams of a protein was hydrolysed into amino acids, one of which is alanine. To this mixture, 0.1 gram of partially deuterated alanine, H2N-CH(CD3)-COOH, was added. After thorough mixing, some of the alanine was separated and purified by crystallization. The crystalline alanine contains 0.652 weight percent of D. How many grams of alanine were originally present in 5 grams of protein? (6)
(b) What is the role of ammoniacal buffer in the volumetric titration of zinc sulphate against EDTA, using Solochrome black (Eriochrome black T) indicator? Write the structure of Zn-EDTA anionic complex. (9)
(a) Calculate the pH of a solution obtained by mixing 50.00 mL of 0.20 M weak acid HA (Ka = 10-5) and 50.00 mL of 0.20 M NaOH at room temperature. (9)
(b) One mole of a salt of type MX is dissolved in 1.00 kg of water. The freezing point of the solution is -2.4 C. Calculate the percent dissociation of the salt in water. (6)
(a) The rate constant of the reaction ClO + NO Cl + NO2 varies with temperature as:
T (K) 200 400
k (cm-3s-1) 2.0x10-11 1.0x10-11
Determine the Arrhenius activation energy for the reaction, assuming that the frequency factor does not change in this temperature range. (6)
(b) Ozone seems to be formed in the atmosphere through the photolysis of diatomic molecule:
Applying steady-state approximation, determine the rate law for the formation of ozone. Show that the formation of ozone follows first order kinetics when the concentration of O3 is extremely small. (9)
42. (a) The reaction N2 + 3 H2 2 NH3 is carried out at 300 K by mixing N2 and H2. The standard free energy of formation of NH3 is -16.4 kJ/mol. After one hour of mixing, the partial pressures of N2, H2 and NH3 are 50 bars, 2 bars and 200 bars, respectively. What is the reaction free energy at this stage of the reaction? (6)
(b) Plot schematically the concentration dependence of molar conductivity of a strong electrolyte and a weak electrolyte in the same figure. The limiting ionic molar conductivities of K+ and Cl- are 73.5 and 76.5 S cm2 mol-1, respectively. If the molar conductivity of 0.1 M KCl solution is 130.0 S cm2 mol-1, calculate the Kohlrauschs constant for KCl solution. (9)
43. (a) The electronic wavefunction (y) for hydrogen atom in the 2s state is given as
f
Determine the most probable radial distance for the electron in this state and also the position of the node (in terms of ao). (9)
(b) Calculate the wavelength corresponding to the lowest energy excitation of an electron confined to a one-dimensional box of length 1 nm. (Energy levels for a particle-in-a-one-dimensional box are given by En = n2h2/8ma2). (6)
(a) Solve the differential equation y " - 5y ' + 6y = 0 with the initial conditions y (0) = -1 and y ' (0) = 0. Here y ' and y " refer to the first and the second derivatives, respectively, of y with respect to x. Verify your answer. (9)
(b) For a particle with position r = 2i - 3j + k and momentum p = i + 2j - 2k in m and kg.m.s-1, respectively, calculate the magnitude of the angular momentum L = r x p.
(6)
12
Add comment
| Earning: Approval pending. |
|
|