Indian Institute of Technology Guwahati (IIT-G) 2005 JAM Chemistry - Question Paper
JAM 2005 Chemistry
|
2005 - CH A
2005 - CH READ THE INSTRUCTIONS ON THE LEFT SIDE OF THIS PAGE CAREFULLY Test Paper Code : CH Time : 3 Hours Max. Marks : 300 INSTRUCTIONS 1. The question-cum-answer book has 32 pages and has 44 questions. Please ensure that the copy of the question-cum-answer book you have received contains all the questions. 2. Write your Roll Number, Name and the name of the Test Centre in the appropriate space provided on the right side. 3. Write the answers to the objective questions against each Question No. in the Answer Table for Objective Questions, provided on page No. 11. Do not write anything else on this page. 4. Each objective question has 4 choices for its answer: (A), (B), (C) and (D). Only ONE of them is the correct answer. There will be negative marking for wrong answers to objective questions. The following marking scheme for objective questions shall be used : (a) For each objective question, you will be awarded 3 (three) marks if you have written only the correct answer. Do not write your Roll Number or Name anywhere else in this question-cum-answer book. (b) In case you have not written any answer for a question you will be awarded 0 (zero) mark for that question. (c) In all other cases, you will be awarded -1 (minus one) mark for the question. I have read all the instructions and shall abide by them. 5. Answer the subjective question only in the space provided after each question. 6. Do not write more than one answer for the same question. In case you attempt a subjective question more than once, please cancel the answer(s) you consider wrong. Otherwise, the answer appearing later only will be evaluated. Signature of the Candidate 7. All answers must be written in blue/ black/blue-black ink only. Sketch pen, pencil or ink of any other colour should not be used. 8. All rough work should be done in the space provided and scored out finally. I have verified the information filled by the Candidate above. 9. No supplementary sheets will be provided to the candidates. 10.Logarithmic Tables / Calculator of any kind / cellular phone / pager / electronic gadgets are not allowed. 11.The question-cum-answer book must be returned in its entirety to the Invigilator before leaving the examination hall. Do not remove any page from this book. Signature of the Invigilator |
| ||||||||||||||||
NOTE : Attempt ALL the 44 questions. Questions 1-30 (objective questions) carry three marks each and questions 31-44 (subjective questions) carry fifteen marks each.
Write the answers to the objective questions ONLY in the Answer Table for Objective Questions provided on page 11.
1. Arrange the following in the decreasing order of acidity of the hydrogen indicated in italics.
|
(l) |
CH3COCH3 |
|
(ii) |
CH3COCtf2COCH3 |
|
(iii) |
CH3OOCCH2COOCH3 |
|
(iv) |
CH3C0Ctf2N02 |
|
(A) |
(ii) > (iii) > (i) > (iv) |
|
(B) |
(iv) > (ii) > (iii) > (i) |
|
(C) |
(iv) > (iii) > (ii) > (i) |
|
(D) |
(ii) > (iv) > (iii) > (i) |
2. For the reaction shown below if the concentration of KCN is increased four times, the rate of the reaction will be
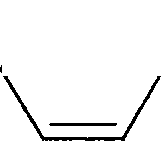
CN + KC1

-Cl + KCN Me ' ' Me
(A) doubled
(B) increased four times
(C) unaffected
(D) halved
3. Benzyl chloride is reacted with different nucleophiles shown below. Arrange them in the decreasing order of reactivity.
Nucleophiles : HOf CH3COO? PhO,0 CH3O0
(A) CH30G > HO0 > PhOQ > CHgCOO0
(B) HO0 > CH3Oe > PhO > CH3COO0
(C) HO > PhO > CH30G> CH3COO
(D) CH3COO > CH3O > HO > PhO
4. The rate of nitration of the following aromatic compounds decreases in the order
(i) benzene
(ii) pyridine
(iii) thiophene
(iv) toluene
(A) (iv) > (i) > (iii) > (ii)
(B) (iii) > (iv) > (i) > (ii)
(C) (iii) > (ii) > (i) > (iv)
(D) (ii) > (i) > (iv) > (iii)
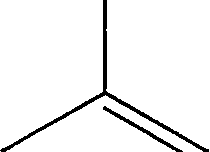
5. The major product formed in the reaction of 1,3-butadiene with bromine is
(A) BrCH2CH(Br)CH=CH2
(B) CH2 = CHCH2CH2Br
(C) CH2=C(Br)C(Br)=CH2
(D) BrCH2CH=CHCH2Br
The reaction of (+) 2-iodobutane and Nal* (I is radioactive isotope of iodine) in acetone was studied by measuring the rate of racemization (kr) and the rate of incorporation
of r(k,).
(+)CH3CH(I)CH2CH3+NaI* - CH3CH(i )CH2CH3 + Nal
For this reaction, the relationship between kr and k is
(A) kj = 2 x kr
(B) ks = (l/2)xkr
(C) kt = kr
(D) ki=(l/3)xkr
Ba(OH)2 DNA - (P)
MgO/A
q_ npi
P04 ' + (Q) - (R) + (S) + sugar
In the scheme shown above (P), (Q), (R) and (S) are
(A) (P) = purine bases, (Q) pyrimidine bases, (R) = nucleotides, (S) = nucleosides
(B) (P) = (Q) = nucleotides, (R) = pyrimidine bases, (S) = purine bases
(C) (P) = nucleosides, (Q) = nucleotides, (R) = (S) = purine bases
(D) (P) = nucleotides, (Q) = nucleosides, (R) = pyrimidine bases, (S) = purine bases
O
HH
oc2h5 + h2 o
O
O
(A)
+ C2H5OH
(B)
+ C2H5OH
Ph 18QH
Ph OH
O
o
+ C2H518OH
(C)
(D)
Ph 18QH
Ph OH
9. The product(s) obtained in the following reaction is (are)
O'
D
,OH
|
OH |
 |
(A)
(B)
+ HO
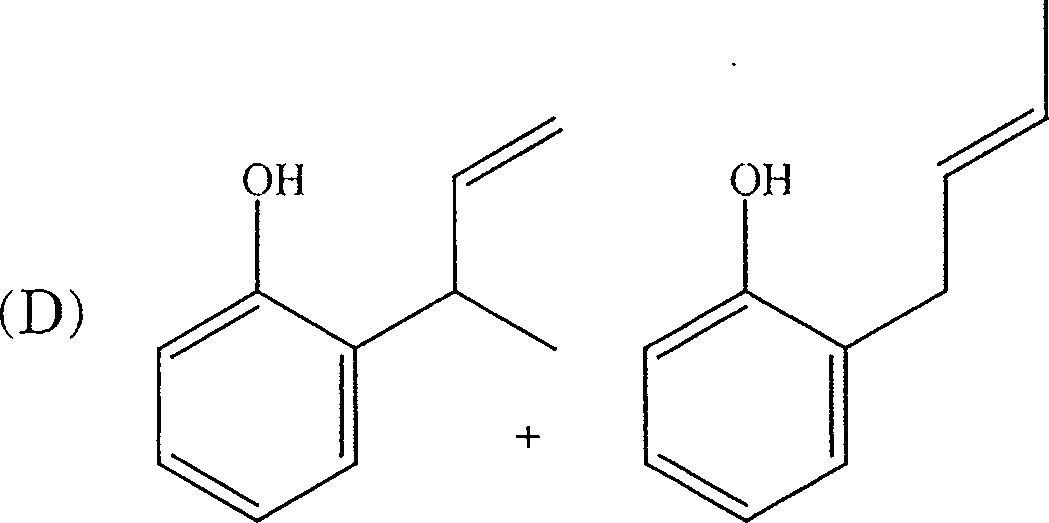
-OH
Amino acid Isoelectric point (X) H2NCH2COOH (I) 9.5 (Y) HOOCCH2CH2CH(NH2)COOH (II) 6.0 (Z) H2N(CH2)4CH(NH2)COOH (HI) 3.1 (A) (X) - (II), (Y) - (III), (Z) - (I)
(B) (X) - (III), (Y) - (I), (Z) - (II)
(C) (X) - (I), (Y) - (II), (Z) - (III)
(D) (X) - (II), (Y) - (I), (Z) - (III)
11. The compound having the highest melting point is
(A) Li Cl
(B) LiF
(C) Li I
(D) LiBr
12. The shape of SF4 is
(A) tetrahedral
(B) trigonal bipyramidal
(C) square planar
(D) octahedral
13. The degree of hydration is expected to be maximum for
(A) Mg2*
(B) Na*
(C) Ba2+
(D) K+
14. The decreasing order of the first ionization energy of the following elements is
(A) Xe > Be > As > A1
(B) Xe > As > A1 > Be
(C) Xe > As > Be > A1
(D) Xe > Be > A1 > As
15. The radioactive isotope used to locate brain tumors is
(A) *D
(B) N
(C) s3 I
(D) *0
16. The crystal field stabilization energy of high spin d' octahedral complex is
(A) A0+2P
5
(B) A0+3P
5
(C) A0+2P
5
(D) A0+3P
5
|
(A) |
|FeF6]3- |
|
(B) |
[MnClJ |
|
(C) |
ICoClJ2 |
|
(D) |
|CoF6f- |
18. On addition of a solution of AgN03 to a solution of Na2S203, it turns black on standing due to the formation of
(A) Ag
(B) Ag2S
(C) Ag2S203
(D) Ag2S04
19. Among the following complexes,
(l) [Ru(bipyridyl)3]+
(ii) [Cr(EDTA)]"
(iii) trans-[CrCl 2 (oxalate )2 ]3~
(iv) cis-[CrCl2 (oxalate )2 ]3~ the ones that show chirality are
(A) (i), (ii), (iv)
(B) (i), (ii), (iii)
(C) (ii), (iii), (iv)
(D) (i), (iii), (iv)
20. The electronic configurations that have orbital angular momentum contribution in an octahedral environment are
(A) d1 and high spin d4
(B) d1 and d2
(C) d2 and high spin d6
(D) high spin d4 and high spin d6
21. For an ideal solution formed by mixing of pure liquids A and B
(B) AHmixis < 0
(O AHmMg > 0
22. The relationship between the equilibrium constant K2 for the reaction :
and the equilibrium constant K2 for the reaction :
(A) 2Kj = K2
(C) K1=K2
23. For H-like atoms, the ground state energy is proportional to
Z2
72
(C) //Z2
where pi is the reduced mass and Z is the nuclear charge.
24. The value of the integral J e~xx2dx is
(B) (x 2e ~x + 2xe ~x + 2e ~x )
25. For the reaction aA products, the plot of -versus time (t) gives a straight line. The
order of the reaction is
(A) 0
(B) 1
(C) 2
(D) 3
26. The pH of a solution prepared from 0.005 mole of Ba(OH)2 in 100 cc water is
(A) 10
(B) 12
(C) 11
(D) 13
27. For an electron whose x-positional uncertainty is 1 x 10~10 m, the uncertainty in the x-component of the velocity in ms-1 will be of the order of
(Data: me = 9 xl0~31 kg, h = 6.6 xl0~34 Js)
(A) 106
(B) 109
(C) 1012
(D) 1015
28. For the following system in equilibrium
CaC03(s) Ca0(s) + C02(g)
the number of components (C), phases (P) and degrees of freedom (F), respectively, are
|
(A) |
2, 2, 2 |
|
(B) |
1,3,0 |
|
(C) |
3, 3, 2 |
|
(D) |
2, 3,1 |
29. For the distribution of molecular velocities of gases, identify the correct order from the following (where v , vav and vrms are the most probable velocity, average velocity and
root mean square velocity, respectively) :
(A) v > v > v
v ' rms y av v mp
(B) v > v > v
v ' mp v rms av
(C) Vav>vrms>vmp () vmp>v,>vrms
30. Given that El = -0.44 V and Ei 3t, 2t = 0.77 V, the E 3t is
Fe / Fe Feci+/Fe+ Fe'r>+/Fe
(A) 1.21 V
(B) 0.33 V
(C) -0.036 V
(D) 0.036 V
Space for rough work
31. Identify the major product(s) formed in the following reactions. Intermediates and reaction mechanisms need not be discussed.
2. H2S04 / A
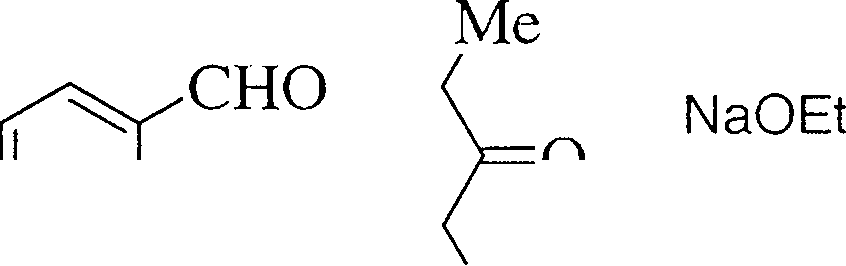 |
|
CHO Me |
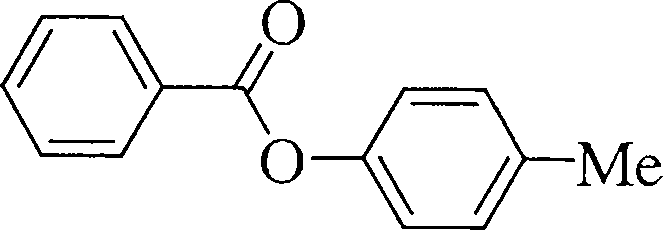
(c) \_/Y /==\ . . 1. HNO3 / H2SQ4 (3)
2. H.O+ / A
32. How may the following transformations be effected? Indicate the reagents / reaction conditions clearly in each step.
(a) (not involving any functional group transformation of the COOH group in the starting material) (6)
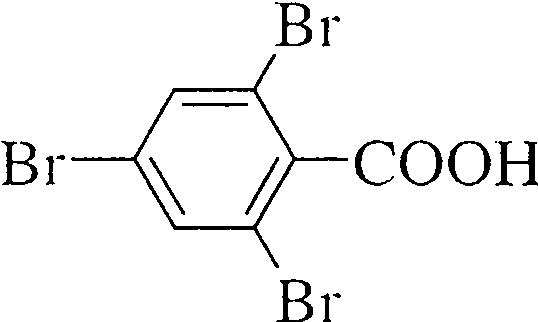
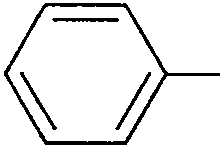 |
COOH |
(b) (using diethyl malonate as the only source of carbon)
(3)
jCOOEt
/
h2cx
ch2cooh
ch2cooh
COOEt
(6)
|
>h |
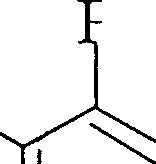 |
Me.
(a) PhCOCH,CH, +Ph-CsC-COOEt Na> l| I (6)
(a) Nitration of 4-i-butyltoluene gives 4-nitrotoluene as one of the products. (3)
(b) cis-4-i-Butylcyclohexyltrimethylammonium hydroxide undergoes Hoffman elimination to yield 4-i-butylcyclohexene whereas the trans isomer does not (use conformations to explain). (6)
1 drv ether
(c) PhMgBr + 2PhCHO --- PhCOPh + PhCH9OH
2. acidic workup
35. (a) Suggest a chemical method for the separation of a mixture containing p-N,N-dimethylaminophenol and p-aminobenzoic acid and give a confirmatory test for phenol. (6)
(b) Write the structures of X, Y and Z in the following. (9)
/V xttt 1- NaNOo / dil. HC1, 0 C
m \ / nh2 --=-x
Uj \=/ z 2. P-naphthol / NaOH
(11) NHMe 'S' ' * I Y
, NaNOo / dil. HC1
(iii) \_y NMe2 --- Z
36. (a) Predict the hybridization and draw the structure of the following molecules based on VSEPRtheory: (i) I3 (ii) S032' (iii) P(CH3)3F2 (9)
(b) Explain why PC15 exists and PH5 does not. (6)
37. (a) Write balanced equations for the formation of (6)
(i) p2074" from PO4" (ii) [(H20)4Fe(0H)2Fe(0H,)4]<+ from [Fe(OH2)6J3+
(b) Which one of the two solutions has lower pH? Justify your answer. (9)
(i) 0.1 M Fe(C104)2 or 0.1 M Fe(C104)3
(ii) 0.1 M Hg(N03)2 or 0.1 M Zn(N03)2
2 + 2 +
38. (a) Between Cu(H20)6 and Co(H20)6 , which one has more distorted structure and why? (6)
(b) Calculate CFSE (in units of A0) and spin only magnetic moment for the following complexes :
(i) [CoF6]3~ (ii) [Fe(CN)6]3~ (iii) [NiCl4]2~ (9)
39. (a) The radioactive element Ra ( Z = 88) emits three alpha particles in succession.
Deduce in which group the resulting element will be found? (6)
(b) A radioisotope sample has an initial activity of 23 dis/min. After j h, the activity is
11.5 dis/min. How many atoms of the radioactive nuclide were present originally? lat1/2=0.69] (9)
40. (a) Write the products of the following reactions : (9)
(i) CH3I + HO" ->
(ii) CF3I + HO ->
(iii) 2 C F3I + Na [Mn (CO)5 ] ->
(b) Arrange BF3 , BC13 and BBr3 in the increasing order of Lewis acidity and justify. (6)
Justify the following : (15)
(a) Considering C02 as an ideal gas, equipartition theorem predicts its total energy as 6.5 kT.
(b) AS for a process is the same whether the process takes place reversibly or irreversibly.
(c) The quantity AG equals the maximum non-expansion work done by a system in a constant temperature-pressure process.
(d) At constant temperature and pressure, AG = 0 for a reversible phase change.
(e) Transition states cannot be isolated as independent chemical species.
42. The rate constant k for a second order reaction P + Q > Products is expressed as
log10 k 20 -~~~~ , where the concentration is in mol lit"1, T is in absolute temperature
and time is in minutes. The initial concentrations of both the reactants are 0.05 M.
Calculate the activation energy and half life of the reaction at 27 C. (R = 2 calK 1 mol 1).
(15)
The equilibrium constant for the reaction
Fe304 (s) + CO(g) ..........3Fe0(s) + C02 (g)
at 600C is 1.00. If a mixture initially consisting of 1 mole of Fe304, 2 moles of CO, 0.5 mole of FeO and 0.3 mole of C02 is heated to 600C at constant total pressure of 5 atmospheres, how many moles of each substance would be present at equilibrium? (15)
44. (a) Use the time-independent Schrdinger equation to calculate the energy of a particle
8 71X 71 y 71 z
of Mass m with V = 0 in the state y/ = J sin-sin- sin- in a cubical box
V a a a a
of length a. (9)
At 20C, the vapor pressure of two pure liquids X and Y which form an ideal solution are 70 torr and 20 torr respectively. If the mole fraction of X in solution is 0.5, find the mole fractions of X and Y in the vapor phase in equilibrium with the solution. (6)
&
A
0

|
Attachment: |
| Earning: Approval pending. |

