Indian Institute of Technology Guwahati (IIT-G) 2005 JAM Biotechnology - Question Paper
JAM 2005 Biotechnology
QUESTION BOOKLET CODE A
Test Paper Code: BT
2005 - BT
DO NOT BREAK THE SEALS ON THIS BOOKLET. AWAIT INSTRUCTIONS FROM THE INVIGILATOR.
Max. Marks: 300
Time: 3 Hours
INSTRUCTIONS:
A. General:
1. This Question Booklet is your Question Paper.
2. This Question Booklet contains 32 pages and has 100 questions.
SEAL
3. The Question Booklet Code is printed on the right-hand top corner of this page.
4. The Question Booklet contains blank spaces for your rough work. No additional sheets will be provided for rough work.
5. Clip board, log tables, slide rule, calculator, cellular phone, pager and electronic gadgets in any form are NOT allowed.
6. Write your Name and Roll Number in the space provided at the bottom.
7. All answers are to be marked only on the machine gradable Objective Response Sheet (ORS) provided, as per the instructions therein.
8. The Question Booklet along with the Objective Response Sheet (ORS) must be
handed over to the Invigilator before leaving the examination hall.
B. Filling-in the ORS:
9. Write your Roll Number in the boxes provided on the upper left-hand-side of the
ORS and darken the appropriate bubble under each digit of your Roll Number using a HB pencil.
10. On the right-hand-side of the ORS, write the Code of the Question Booklet received by you in the box provided, with ball-point pen, and darken the appropriate bubble with HB pencil.
11. On the lower-left-hand-side of the ORS, write your Name, Roll Number, Name of the Test Centre and put your signature in the appropriate box with ball-point pen. Do not write these anywhere else.
C. Marking of Answers on the ORS:
12. Each question has 4 choices for its answer: (A), (B), (C) and (D). Only ONE of them is the correct answer.

13. On the right-hand-side of ORS, for each question number, darken with a
HB Pencil, ONLY one bubble corresponding to what you consider to be the most
appropriate answer, from among the four choices.
14. There will be negative marking for wrong answers.
MARKING SCHEME:
(a) For each question, you will be awarded 3 (three) marks, if you have darkened only one bubble corresponding to the correct answer.
(b) In case you have not darkened any bubble for a question, you will be awarded 0 (zero) mark for that question.
(c) In all other cases, you will be awarded -1 (minus one) mark for the question.
Roll Number | || || || || || |
Name
In a chemical synapse, receptors for neurotransmitters are found on
(A) presynaptic membranes
(B) postsynaptic membranes
(C) synaptic vesicles
(D) myelin sheaths enveloping axons
During an allergic immune response, histamine is released from
(A) B lymphocytes
(B) T lymphocytes
(C) mast cells
(D) special Lymphocytes that also secrete IgE
Prostaglandins are biologically active molecules that are
(A) monocyclic
(B) bicyclic
(C) tricyclic
(D) poly cyclic
When animal cells are placed in a hypotonic solution such as distilled water, they swell and burst due to
(A) diffusion
(B) osmosis
(C) active transport
(D) pinocytosis
Identify the hormone secreted by the pituitary gland that causes the smooth muscle of the uterus to contract during parturition in mammals.
(A) Vasopressin
(B) Oxytocin
(C) Prolactin
(D) Gonadotropins
6. How many antigen-binding sites does a pentameric IgM molecule contain?
(A) Two
(B) Five
(C) Ten
(D) Fifteen
7. Identify the character that is lost by an animal cell when it gets transformed into a cancer cell.
(A) Differentiation
(B) Contact inhibition
(C) Regeneration
(D) Totipotency
Group I
Nucleotide Amino acid Fatty acid Vitamin
1.
2.
3.
4.
5.
6.
P.
Q.
R.
S.
The correct match between Group I and Group II is
Group II
Ascorbic acid Adenosine triphosphate
Aspartic acid Gluconic acid Palmitic acid Uric acid
|
(A) |
P-6, |
Q-3, |
R-4, |
S-l |
|
(B) |
P-2, |
Q-3, |
R-5, |
S-4 |
|
(C) |
P-2, |
Q-3, |
R-5, |
S-l |
|
(D) |
P-6, |
Q-4, |
R-3, |
S-l |
P. Neurotransmitter
Group II
1. Acetylcholine
2. Papain
3. Interferon
4. Streptomycin
5. cAMP
6. Ecdysone
Q. Hormone
R. Second messenger
S. Enzyme
(A) P-l, Q-6, R-5, S-2
(B) P-6, Q-5, R-3, S-2
(C) P-3, Q-6, R-l, S-4
(D) P-l, Q-3, R-5, S-2
10. Enzymes catalyze chemical reactions by
(A) decreasing the activation energy
(B) increasing the activation energy
(C) providing a buffering effect
(D) regulating the concentration of substrates at optimal temperature and pH
11. In a typical ecosystem, biomagnification occurs at the highest level in
(A) primary producers
(B) secondary producers
(C) primary consumers
(D) secondary consumers
12. Western blot is used for the identification of
(A) monosaccharides
(B) RNA
(C) DNA
(D) proteins
13. Which of the following does NOT form the basis of an antigen-antibody binding?
(A) Hydrogen bond
(B) Ionic interactions
(C) Covalent bond
(D) Hydrophobic interactions
14. Myoglobin contains multiple distinct epitopes but only a single copy of each epitope. Identify the condition under which antigen-antibody precipitation reaction would occur.
(A) When monoclonal antibody is used
(B) When specific polyclonal antiserum is used
(C) When monovalent Fab fragments are used
(D) When any of the above conditions are fulfilled
15. According to the taxanomical classifications of humans,
(A) Homo refers to genus and sapiens the species
(B) Homo refers to family and sapiens the genus
(C) Homo refers to order and sapiens the family
(D) Homo refers to class and sapiens the sub-class
16. The optical rotation of a solution of D-glucose is +52.7. Which of the following statements applies to this solution?
(A) It is a mixture of two forms of D-glucose and both forms exhibit the same specific optical rotation
(B) It has only one form of D-glucose and all molecules have the same specific optical rotation
(C) It is a mixture of two forms of D-glucose, each of which has different specific optical rotation
(D) It is a mixture of large number of D-glucose forms, each of which has different specific optical rotation
17. The decreasing order of the melting points of the following fatty acids is P. Stearic acid, 18:0
Q. Cis-oleic acid, 18:1 R. Trans-oleic acid, 18:1 S. Linolenic acid, 18:3
(A) P > Q > R > S
(B) P > R > Q > S
(C) S > R > Q > P
(D) S > Q > R > P
18. Consider the following four statements:
P. The solubility of a protein is lowest at its isoelectric point
Q. At low ionic strengths, solubility of a protein increases with increasing salt concentration
R. Tyrosine, tryptophan and phenylalanine have aromatic side chains capable of forming hydrogen bonds
S. Oxygen binding to hemoglobin decreases when pH is increased from 7.2 to 7.6 Of these statements,
(A) only P and Q are correct
(B) P, Q and S are correct
(C) all are correct
(D) only Q is correct
19. For the enzyme catalyzed reaction
km is an indicator of the affinity of enzyme to the substrate
(A) when k2 k_x
(B) when k2 k
(C) when k2 = k_x
(D) irrespective of the mutual relationship of the rate constants
20. Which of the following statements is FALSE?
(A) Nitrogen fixation by the nitrogenase complex requires eight electrons
(B) Conversion of nitrogen to ammonia (N2 + 3H2 2NH3) is an endergonic process
(C) Certain free living aerobic bacteria are also capable of nitrogen fixation
(D) The nitrogen fixing nitrogenase complex is oxygen-labile
21. Which of the following is NOT an allosteric modulator of hemoglobin?
(A) Carbon dioxide
(B) H+
(C) 2,3-Bisphosphoglycerate
(D) Carbon monoxide
22. Relative to the inter-membrane space, the mitochondrial matrix is
(A) alkaline and has negative membrane potential
(B) acidic and has negative membrane potential
(C) alkaline and has positive membrane potential
(D) acidic and has positive membrane potential
|
(A) |
P680.Chl |
|
(B) |
h20 |
|
(C) |
P680.Chl |
|
(D) |
P700 |
24. One of the carbon atoms of glucose is 14C-labeled. If none of the TCA cycle intermediates are 14C-labeled after glycolysis and one cycle of Krebs cycle, the carbon atom of glucose that was
|
labeled is | |
|
(A) |
Cl |
|
(B) |
C6 |
|
(C) |
C2 |
|
(D) |
C3 |
25. Which of the following statements relating to microtubules is NOT correct?
(A) The plus-end of microtubule is the fast-growing end
(B) Addition of short fragments of microtubules enhances polymerization
(C) A microtubule with GDP-cap enters the shrinkage phase (catastrophe)
(D) Critical concentration for polymerization is same for both plus- and minus-ends
26. Treadmilling of actin filaments refers to
(A) net assembly at both plus- and minus-ends
(B) net assembly at plus-end and net disassembly at minus-end
(C) net disassembly at plus-end and net assembly at plus-end
(D) net disassembly at both plus- and minus-ends
27. Which of the following vitamins becomes part of a high-energy metabolite in the cell?
(A) Thiamine
(B) Riboflavin
(C) Pantothenate
(D) Folate
28. Which of the following four is involved in cell cycle control?
(A) Proteolysis of cyclins
(B) Phosphorylation of cyclins
(C) Proteolysis of cyclin-dependent kinases
(D) Dephosphorylation of cyclins
29. Which of the following is the key reaction linking carbon and nitrogen cycles?
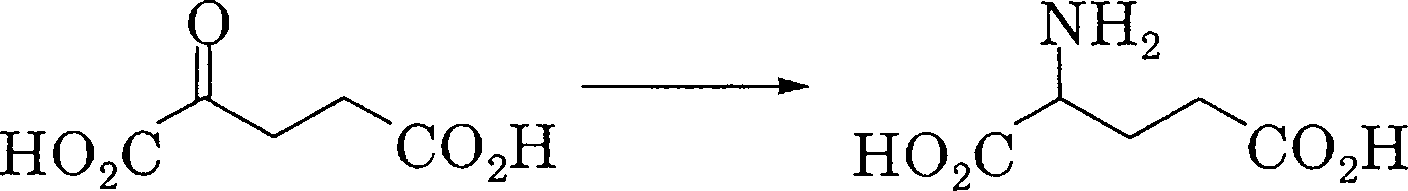
(A)
(B)
|
nh2 |
 |
O
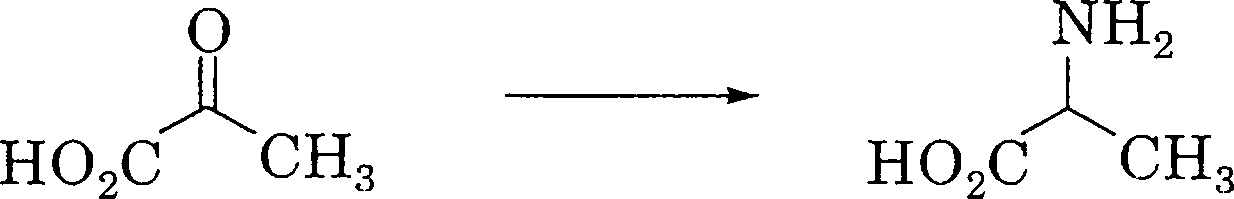
ho2c h
30. The most effective enzyme-catalyzed reaction is the one in which
(A) kcat = 1.4 x 104 sec-1 and km = 9 x 10-5
(B) kcat = 1.4 x 105 sec-1 and km = 9 x 10-5
(C) kcat = 1.4 x 103 sec-1 and km = 9 x 10-5
(D) kcat = 1.4 x 104 sec-1 and km = 9 x 10~4
31. The deoxyribonucleotides in a DNA strand are linked together covalently through
(A) 5-hydroxyl group of one sugar and 5-phosphate group of the next
(B) 2-hydroxyl group of one sugar and 3-hydroxyl group of the next
(C) 3-hydroxyl group of one sugar and 5-phosphate group of the next
(D) 5-hydroxyl group of one sugar and 3-hydroxyl group of the next
32. sno-RNAs
(A) process and chemically modify ribosomal RNAs
(B) are involved in telomere synthesis
(C) are involved in splicing pre-mRNA
(D) form the basic structure of ribosome
33. How many bands would you expect if a pentameric sample of IgM containing |3-mercaptoethanol is subjected to SDS-PAGE?
(A) 2
(B) 3
(C) 5
_A
34. In DNA-gel retardation assay, which of the following complexes that are formed is analyzed?
(A) DNA-RNA complex
(B) DNA-DNA complex
(C) RNA-protein complex
(D) DNA-protein complex
35. The TATAa/tAa/t sequence, present in the eukaryotic promoter, is recognized and initially bound by which of the following transcription factors?
(A) TFIIA
(B) TFIIB
(C) TFIID
(D) TFIIH
36. The backbone of peptidoglycan present in the bacterial cell wall is a polysaccharide consisting of repeating units of
(A) N-acetylgalactosamine - N-acetylmuramic acid
(B) N-acetylgalactosamine N-acetylneuraminic acid
(C) N-acetylglucosamine - N-acetylmuramic acid
(D) N-acetylglucosamine N-acetylneuraminic acid
37. Which of the following statements is true with respect to the influenza virus?
(A) Hemagglutinin present in the virus envelope is involved in attachment of the virus to sialic acid residues of the host cell surface
(B) Hemagglutinin present in the virus envelope is involved in the attachment of the virus to N-acetylglucosamine residues of the host cell surface
(C) Hemagglutinin proteins form tetramers that project out from viral surface
(D) Hemagglutinin is not a glycoprotein
(A) Helicobacter pylori
(B) Hemophilus influenzae
(C) Vibrio fischeri
(D) Naegleria fowleri
39. Choose the right combination from the following statements with respect to proof reading activity during DNA replication.
P. DNA polymerase activity is involved
Q. DNA topoisomerase I activity is involved
R. 3 5-exonuclease activity is involved
S. 5 > 3-exonuclease activity is involved
(A) PQ
(B) PR
(C) PS
(D) QR
40. Which one of the following movements of phospholipids is catalyzed by the phospholipid translocators in the eukaryotic cell membrane?
(A) Lateral
(B) Flexion
(C) Flip-flop
(D) Rotation
41. Baculovirus vectors are used to transfer genes into
(A) mammalian cells
(B) plant cells
(C) insect cells
(D) bacterial cells
42. Choose the right combination from the following statements with respect to the amino acid activation during protein biosynthesis.
P. A single aminoacyl-tRNA synthetase can catalyze the coupling of all amino acids to appropriate tRNAs
Q. The energy during the activation step is provided through GTP hydrolysis
R. The energy during the activation step is provided through ATP hydrolysis
S. The aminoacyl-tRNA synthetase catalyzed reaction attaches amino acid to the 3-end of the tRNA.
(A) PR
(B) PS
(C) QS
(D) RS
Space for rough work
|
P. DNA ligase Q. DNA primase R. DNA topoisomerase I S. DNA helicase |
1. Unwinds dsDNA into ssDNA 2. Synthesizes small DNA fragments as primers 3. Joins 3-OH and 5-phosphate ends of DNA 4. Produces a transient single strand break in the phosphodiester backbone of DNA 5. Synthesizes RNA primers 6. Converts ssDNA into dsDNA |
|
(A) |
P-3, |
Q-2, |
R-6, |
S-l |
|
(B) |
P-3, |
Q-5, |
R-4, |
S-l |
|
(C) |
P-5, |
Q-2, |
R-l, |
S-3 |
|
(D) |
P-4, |
Q-2, |
R-3, |
S-6 |
44. Which of the following statements is NOT correct with respect to elongation step of prokaryotic protein biosynthesis?
(A) fMet-tRNAf is recognized by EF-Tu-GTP
(B) Binary complex of EF-Tu-GTP binds aminoacyl-tRNA to form a ternary complex
(C) Binary complex EF-Tu-GDP is inactive
(D) Kirromycin is an antibiotic that inhibits the function of EF-Tu
45. If the ionization energy of H is 13.59 eV, then the ionization energy of He+ will be
(A) 13.59 eV
(B) 27.18 eV
(C) (13.59)2 eV
(D) 54.36 eV
46. In a C02 molecule the number of translational, rotational and vibrational degrees of freedom, respectively, is
(A) 3, 2, 4
(B) 3, 4, 2
(C) 3, 3, 3
(D) 4, 3, 2
47. The molecule which is IR inactive and Raman active is
(A) HC1
(B) N2
(C) S02
(D) C02
48. Which of the following axis of symmetry does the tetragonal crystal possess?
(A) Two fold
(B) Three fold
(C) Six fold
(D) Four fold
|
(A) |
N2 + 02 -> 2NO |
|
(B) |
C + i 02 -4 CO |
|
(C) |
C + O2 - C02 |
|
(D) |
N2O4 + V2 O2> N2O5 |
50. Assuming that AHvap is 540 kcal g 1, what would be the AS accompanying the evaporation of one mole of water at 100C?
(A) 540 cal g_1
(B) 25 cal K'W1
(C) 1.45 cal Kmol"1
(D) 26.06 cal ICW'1
51. For a reaction 2A + B > P, by doubling the initial concentration of both the reactants the rate increases by a factor of 8, whereas by doubling the concentration of B alone the rate increases two times. The rate law for the reaction is
at
at
at
dt
52. For the Zn-Cu cell, E = 1.10 V. If the reduction potential of the Cu2+(aq)/Cu(s) couple is
0.34 V, then that of the Zn2+ (aq)/Zn (s) couple is
(A) -0.76 V
(B) 0.76 V
(C) 1.44 V
(D) 1.10 V
53. The geometry of the complex [Ni (CN)4 ]2~ is
(A) Tetrahedral
(B) Square planar
(C) Octahedral
(D) Distorted tetragonal
54. The increasing order of stability of 022-, 02_, 02, 02+ species is
(A) 02+, 02", 022", 02
(B) 022", 02-, 02, 02+
(D) 02+, 022, 02', 02
55. The average distance of an electron from the nucleus in the ground state of hydrogen atom (in units of Bohr radius a0 ) is
(A) 1
(B) 2
(C) 3/2
56. Phthalimide on treatment with base will undergo Hoffman rearrangement to give
(A) aniline
(B) benzylamine
(C) 2-aminobenzoic acid
(D) 3-aminobenzoic acid
57. Which one of the following compounds is antiaromatic?
(A) Cyclopentadiene
(B) Cyclobutadiene
(C) Azulene
(D) Cycloheptatrienyl cation
58. Choose the correct absolute configuration for the following compound.
-Br
H---Br
Br--H
CH3
(C) 2S, 3S
59. Nylon-6,6 is made from
(A) caprolactam
(B) adipic acid and hexamethylenetetramine
(C) phenol and formaldehyde
(D) terephthalic acid and ethylene glycol
60. Which one of the following is not a heterocyclic amino acid?
(A) Proline
(B) Tyrosine
(C) Histidine
(D) Tryptophan
61. Carbylamine test is usually carried out to confirm the presence of
(A) nitro group
(B) primary amine
(C) secondary amine
(D) tertiary amine
62. The following reduction is best achieved by
Me C02Me
Me = C02Me- )=K
H H
(A) H2/Pd-C
(B) H2/Lindlar catalyst
(C) LiAlH4
(D) Na/liq. NHs
|
(A) | 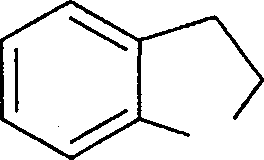 |
|
N H | |
|
(B) | 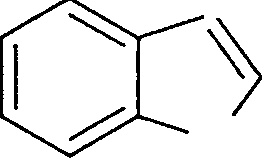 |
|
N H | |
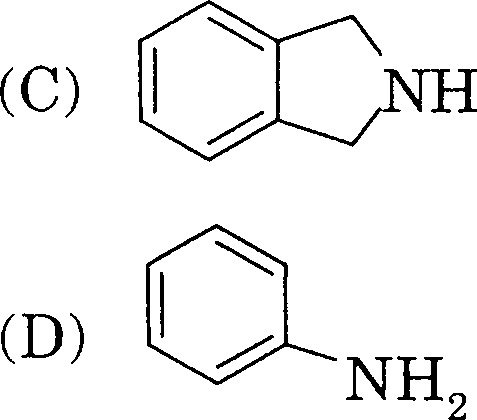
64. The correct match between Group I and Group II is
|
Group I P. AlCls Q. Salicylaldehyde R. 2-Allylvinylether S. 2-bromobutane |
Group II 1. Cope rearrangement 2. Sn2 reaction 3. Friedal-Crafts reaction 4. Reimer-Teiman reaction 5. Claisen rearrangement 6. Kolbe reaction |
|
(A) |
P-3, |
Q-5, |
R-l, |
S-2 |
|
(B) |
P-2, |
Q-4, |
R-5, |
S-6 |
|
(C) |
P-3, |
Q-4, |
R-l, |
S-2 |
|
(D) |
P-3, |
Q-4, |
R-5, |
S-2 |
|
Three lines y = |
0, y |
= X | ||
|
(A) |
8 sq. |
units | ||
(B) 16 sq. units
-4 constitute a triangle. Its area is
(C) - 8 sq. units
(D) - 16 sq. units
, 2 1 2/3
dx
k
dx2
are, respectively
(A) 2,2
(B) 2,3
(C) 3,2
(D) 2,4
67. For what value of p , the vectors 2i - j + k , i + 2j - 3k and 3i + pj + 5/e are coplanar?
(A) 4
(B) 0
(C) 22/5
(D) -4
68. If f(x) = 3, when - 3 < x < -1 = -6x - 3, when - 1 < x < 0 = 3x - 3 , when 0 < x < 1, then the values of x for which 2f(x) + 3 = 0 are
(A) 1/4, 1/2
(B) - 1/4 , 1/2
(C) 1/4, -1/2
(D) -1/4, -1/2
|
1 0 1 |
X | |
|
1 1 1 OJ i |
5 |
|
(A) |
7,-3 |
|
(B) |
-7,3 |
|
(C) |
-7, -3 |
|
(D) |
7,3 |
70. A, B and C toss a coin in succession on the understanding that the first one to throw a head wins. The probability that C wins is
(A) 1/7
(B) 2/7
(C) 4/7
(D) 1/8
71. If xy =ex~y, then is
dx
(A) log*
Hog(ex)]2
[log(ex)]
(C)
[log(ex)]2
xlogx
[logiex)}
72. The shortest distance between the lines
x-1 y-2 z-3 , x-2 y-4 z-5 .
- = -- = - and - = -- = - is
2 3 4 3 4 5
(A) l/Vi
(B) 5/Vi
(C) ll/V6
(D) 15/V6
73. The curves 2x2 +3y2 =1 and px2 + 6y2 =1 intersect orthogonally if p is
(A) 1/3
(B) 3
(C) 4
(D) 2/3
74. If the profit function p (x) = 41 - 24x - 18x2 , then the maximum profit that a company can make is
(A) 49
(B) 65
(C) 33
(D) 17
75. A particle is in equilibrium under the action of three forces P, Q and R. If the angle between P and Q is 120 and that between Q and R is 135, then the ratio of their magnitudes P : Q : R is
(A) 2 : V3 + 1 : V6
(B) 2 : V3 +1 : V2
(C) V6:V3+1:2
(D) 2 : V6 : a/3 + 1
76. A man can throw a stone to a maximum distance of 50 m. The time in seconds for which the stone remains in the air is
(B) 5/4g
(D) 5/(2Vi)
77. If A + S = #/4, then (l + tan A) (l + tan B) is equal to
(A) 1
(B) 2
(C) V3
(D) 0
78. If 1, w, w2 are the cube roots of unity, then the value of (l + w)z - (l + w2 )3 is
(A) 2 w
(B) 2
(C) -2
(D) 0
79. If the arithmetic mean of the roots of a quadratic in x is 8 and geometric mean is 5, then the quadratic is
(C) x2 - 16x + 25 = 0
|
(A) |
251 |
|
(B) |
250 |
|
(C) |
249 |
|
(D) |
0 |
22 23 24
81. If x = 3 +--1---1--+ ... oo, then 1/x is equal to
2! 3! 4! /h
|
(A) |
<T2 |
|
(B) |
e2 |
|
(C) |
e1/2 |
|
(D) |
e~1/2 |
n+2 y
82. If ;- = , then the value of n is
n~PA 48
(A) 7
(B) 6
(C) 5
(D) 4
83. The displacement x of a particle as a function of time t is the figure. The acceleration of the particle is
|
x | 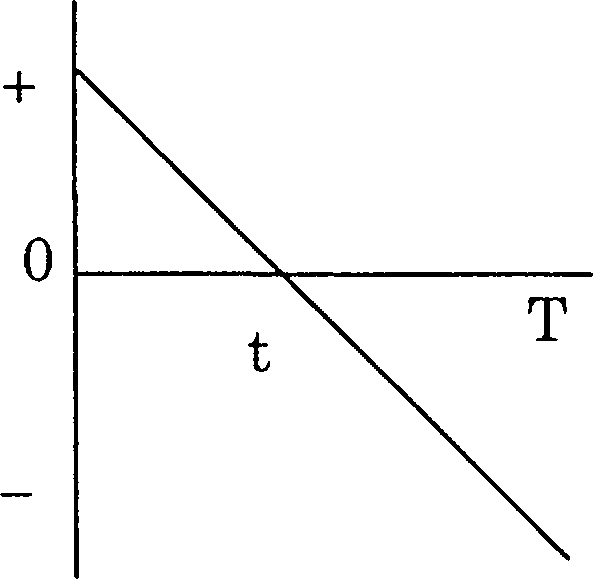 |
given in
(A) always positive
(B) always negative
(C) always zero
(D) positive during time 0 to t and negative during t to T
84. A ball is dropped from a height of 20 m. After its impact with the ground, it rebounds with a velocity half of its velocity just before the impact. The maximum height reached after impact is
(A) 20 m
(B) 15 m
(C) 10 m
(D) 5 m
85. The electric potential at a distance r, far off from an electric dipole of moment p, is
/ \
1
p. r
(A)
47T(
/ \
4jZr
p.r
(B)
/ N\ p. r
(C)
(D)
4 7lf
r \ > > pxr
4:718 r
86. An ideal heat engine operating between a source and an intermediate sink has 50% efficiency. Another ideal heat engine operating between this intermediate sink and a final sink has 40% efficiency. The efficiency of an ideal engine operating between the same source and same final sink is
(A) 90%
(B) 70%
(C) 45%
87. A current of 20 A flows through a copper rod of diameter 2 cm. The magnetic field at a distance 5 mm from the axis of the rod is
(A) xlO3 T
2 71
(B) x 10s T
2 n
(C) x 103 T
2 71
2 71
88. A thin convex lens is placed between a mirror and an object in such a way that the image of the object falls on to itself. The separation between the object and the lens is 20 cm. Now, if another thin lens is placed between mirror and convex lens in contact with the convex lens, the object is to be moved by 10 cm away from convex lens in order that the image of the object falls on to itself. The new lens is a concave lens of focal length
|
(A) |
10 |
cm |
|
(B) |
30 |
cm |
|
(C) |
40 |
cm |
|
(D) |
60 |
cm |
89. The half life of two elements A and B is 5 and 15 yrs, respectively. If equal numbers of atoms of A and B are present in an alloy initially, then the time when the number of B atoms is twice that of A atoms is
|
(A) |
2.5 |
yrs |
|
(B) |
7.5 |
yrs |
|
(C) |
10 |
yrs |
|
(D) |
20 |
yrs |
90. An optical fibre has a core of diameter 20 jim and refractive index 1.5. If the fibre has maximum entrance angle of 60, the refractive index of the cladding is
(B) V3/2
91. The SI unit of the permittivity of free space e0 is
(A) m-3 kg-VA2
(B) m-2 kg-1s4A2
(C) m-3 kg-1s4A-2
(D) m-2 kg-1s4A~2
92. A meter bridge is used for the measurement of
(A) length
(B) potential
(C) resistance
(D) current
93. The role of graphite in a nuclear reactor is
(A) to increase the energy of neutrons
(B) to decrease the energy of neutrons
(C) to act as a catalyst in uranium fission
(D) to act as a source of neutron
94. In a PNP transistor, base is at a
(A) lower potential than emitter
(B) higher potential than emitter
(C) lower potential than collector
(D) higher potential than both collector and emitter
95. The variation of resistivity p as a function of temperature T is shown in the figure.
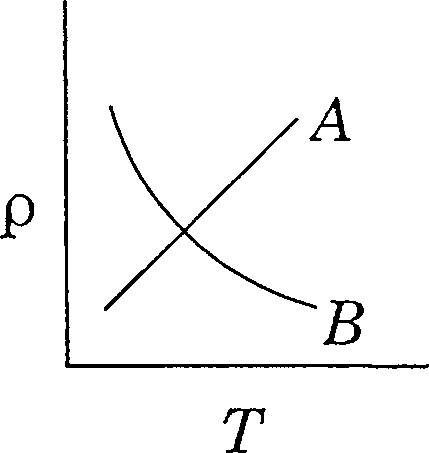
(A) Curve A is for metal and B for a semiconductor
(B) Curve A is for semiconductor and B for metal
(C) Curve A is for undoped semiconductor and B for doped semiconductor
(D) Curve A is for doped semiconductor and B for undoped semiconductor
96. A disk of mass m and radius R/2 is attached to a disk of mass M and radius R as shown
in the figure. The moment of inertia of the system about an axis passing through center of the bigger disk and perpendicular to the disk is
(A) -MR2 +-m(R/2f
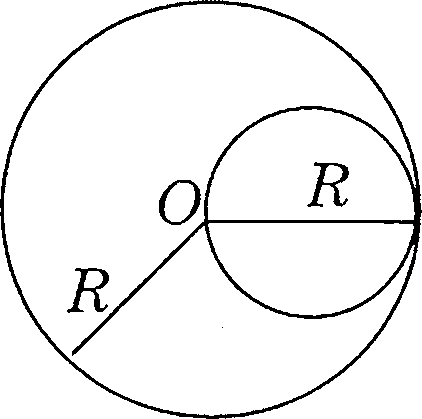
2 2
(B) -MR2 +-?n(R/2)2
(D) (M+m)R2
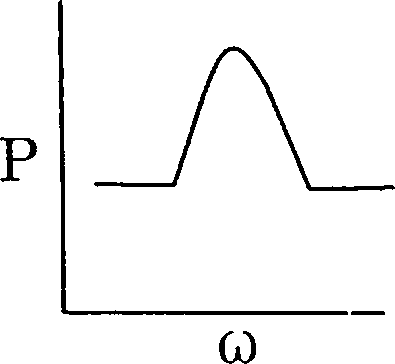
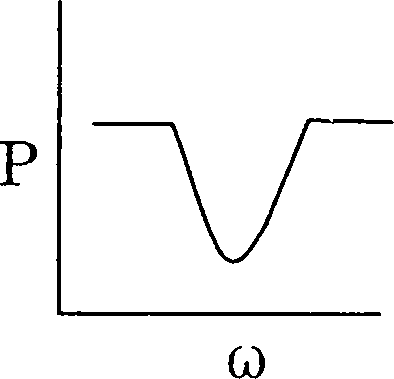
97. A series LCR circuit is driven with a constant voltage ac source of variable frequency co. The power P dissipated in the circuit is graphically represented by
(A)
(B)
|
(0 |
 |
(D)
98. A series combination of two capacitors C1 and C2 is charged by a voltage source. Cx is then discharged through an electrolytic cell liberating m1g of a metal. Now, the parallel combination of C1 and C2 is charged by the same source and Cx is discharged through the same cell liberating m2 g of the metal. The ratio is
(A) (C.+C/C,
(B) (C,+C2)/C2
(C) Cj/(Cj + C2)
99. The intensity at the mid point on the line connecting two light sources has a maximum value of 36 units. At a point x distance (from the mid point) towards either source, it has a minimum value of 4 units. The intensity at a distance .x/2 is
(A) 25 units
(B) 20 units
(C) 12 units
(D) 6 units
100. Two electric bulbs rated 120 W and 80 W are connected in series to mains (240 V) supply. The net power consumed is closest to
(A) 200 W
(B) 80 W
(C) 50 W
(D) 40 W
Space for rough work

|
Attachment: |
| Earning: Approval pending. |
