Indian Institute of Technology New Delhi - (IIT) 2010 M.Sc BioTechnology Iit jam entrance for biotech - Question Paper
2010 - BT D
QUESTION BOOKLET CODE Test Paper Code : BT
2010 - BT D
Max. Marks: 300
Time: 3 Hours
INSTRUCTIONS
A. General:
1. This Question Booklet is your Question Paper.
2. This Question Booklet contains 24 pages and has 100 questions.
3. The Question Booklet Code is printed on the right-hand top corner of this page.
4. The Question Booklet contains blank spaces for your rough work. No additional sheets will be provided for rough work.
5. Clip board, log tables, slide rule, calculator, cellular phone or electronic gadgets in any form are NOT allowed.
6. Write your Name and Registration Number in the space provided at the bottom.
7. All answers are to be marked only on the machine gradable Optical Response Sheet (ORS) provided along with this booklet, as per the instructions therein.
8. The Question Booklet along with the Optical Response Sheet (ORS) must be handed over to the Invigilator before leaving the examination hall.
B. Filling-in the ORS :
9. Ensure that the code on the Question Booklet and the code on the ORS are the same. If the codes do not match, report to the Invigilator immediately.
10. Write your Registration Number in the boxes provided on the upper left-hand-side of the ORS and darken the appropriate bubble under each digit of your Registration Number using a HB pencil.
11. On the lower-left-hand-side of the ORS, write your Name, Registration Number, Name of the Test Centre and put your signature in the appropriate box with ballpoint pen. Do not write these anywhere else.
C. Marking of Answers on the ORS :
12. Each question has 4 choices for its answer : (A), (B), (C) and (D). Only ONE of them is correct.
13. On the right-hand-side of ORS, for each question number, darken with a HB Pencil ONLY one bubble corresponding to what you consider to be the most appropriate answer, from among the four choices.
14. There will be negative marking for wrong answers.
MARKING SCHEME:
(a) For each correct answer, you will be awarded 3 (Three) marks.
(b) Multiple answers to a question will be treated as a wrong answer.
(c) For each wrong answer, you will be awarded -1 (Negative one) mark.
(d) For each un-attempted question, you will be awarded 0 (Zero) mark.
|
Name | |||||||
|
Registration Number | |||||||
D
&
o
Q. 1 The composition, of proteins Pi to P4 are shown below:
Protein Composition
Pi Rich in polar residues; poor in apolar residues P2 Rich in apolar residues; poor in polar residues P3 Has comparable number of polar and apolar residues
P4 Rich in glycine and proline
Which one of the following options CORRECTLY relates the propensities of these proteins to be folded, aggregated or disordered in an aqueous buffered solution?
(A) Pi, P2 and P4 are disordered and P3 is folded
(B) Pi and P3 are folded, P2 is aggregated and P4 is disordered
(C) Pi and P3 are folded, and P2 and P4 are disordered
(D) Pi and P4 are disordered, P2 is aggregated and P3 is folded
|
Q.2 EnzP, EnzQ, EnzR, and EnzS catalyze the metabolic reactions as shown below: |
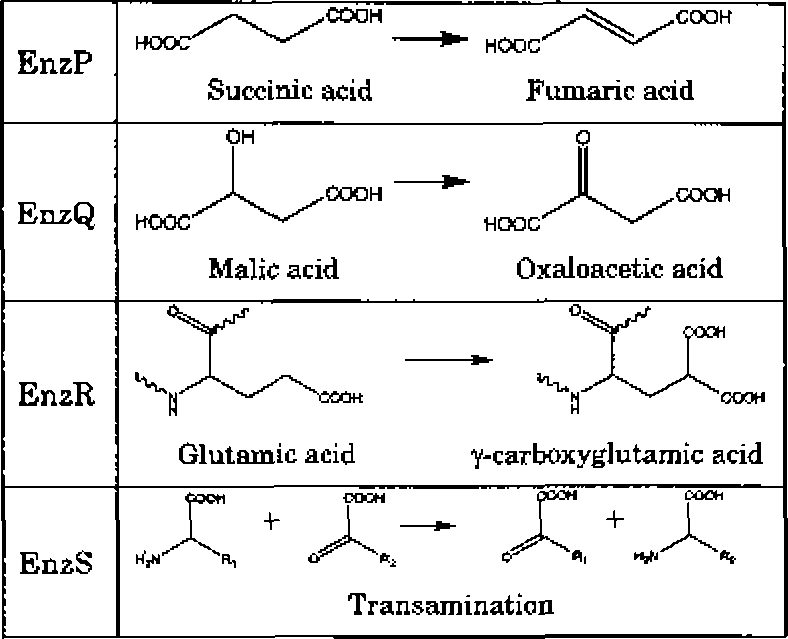 |
Each of the above enzymes is dependent on one of the following four vitamins (either the vitamin itself or its derivative):
VitB2: Vitamin B2 (riboflavin)
VitB3: Vitamin B3 (niacin)
VitB6: Vitamin B6 (pyridoxal)
VitK: Vitamin K
Which one of the following options gives the CORRECT enzyme-vitamin matches?
(A) EnzP and VitB3, EnzQ and VitB2, EnzR and VitB6, EnzS and VitK
(B) EnzP and VitB2, EnzQ and VitB3, EnzR and VitB6, EnzS and VitK
(C) EnzP and VitB2, EnzQ and VitB3, EnzR and VitK, EnzS and VitBG
(D) EnzP and VitB6, EnzQ and VitB2, EnzR and VitB3, EnzS and VitK
D
Q.3 The ground state energy of hydrogen atom is -13.6 eV. Assume he = 1240 eV.nm. The maximum wavelength in Baimer series (in nm) is approximately
(D) 653
(C) 244
(B) 122
(A) 103
, at a higher pressure P and temperature 71, is suddenly
Q.4 A sample of gas
= 1.5
sCv
released to atmosphere. The final temperature of the gas is . The value of P (in the
units of aim) is
(A) 4 (B) 4V2 (C) 8 (D) 8V2
Q.5 A ball is thrown with a speed of 40 m/sec in a direction of 30* with the ground. Assume g = 10 m/sec2. The ball will reach to a maximum height (in meters) of
(A) 20
(C) 60
(B) 40
(D) 80
Q.6 The dimensions MJ}T 3 DO NOT correspond to
(A) work (C) heat
(B) torque
(D) angular momentum
n
n
Q7
lim
13
n (n+l)d (n + 2 Y
(C, |
(A) 8
(B) 4
(D)
Q.S The area (in square units of length) enclosed by = 2* 2+1 and 6x-y = 3 is
(C) 1
<B> I
<D> !
<*> ;
Q.9 Suppose the principal increases continuously at the rate of r% per year. If Rs. 100 doubles in 10 years, then r is
Q. 10 A eukaryotic cell lacking active telomerase
(A) will be unable to proofread incorrectly-added nucleotides
(B) is highly probable to be a cancerous cell
(C) will experience a gradual reduction of chromosome length with each replication cycle
(D) will be unable to connect Okazaki fragments
Q.31 Which of the following are used as reporter genes?
P. -glucuronidase gene
Q. Ampicillin-resistance gene
R. Gal4 gene
S. Luciferase gene
(A) PandS (B) P and Q <C) RandS (D) Q and R
Q.12 p-Aminobenzoic add is a biosynthetic precursor of
(A) glutamic acid (B) acetic acid (C) citric acid (D) folic acid
Q.13 Ribosomes are made of
(A) DNA and proteins (C) only proteins
(B) RNA and proteins (D) DNA, RNA and proteins
Q.14 The anticodon in a tRNA is
(A) complementary to codon in rRNA
(b) complementary to codon in mRNA
(C) complementary to 3'-end of tRNA where amino add binds
(D) changeable depending upon the amino acid it binds to
Q.15 Which one of the following hormones shows photoperiodidty?
(A) Thyroxine (B) Melatonin (C) Cortisol (D) Relaxin
Q.1G Which of the following ligament(s) is/are attached to ovary?
P. Ovarian ligaments
Q, Suspensory ligaments
R. Broad ligaments
(A) OnlyP (B) Only P and Q (C) Only P and R (D) P, Q and R
Q.17 The role of salicylic acid in systemic acquired resistance of plants is to
(A) directly destroy the pathogens
(B) activate defenses throughout the plant before the infection spreads
(C) activate heat shock proteins
(D) sacrifice the infected tissue
|
Q.18 Match the therapeutics in Column I with their applications in Column II. | ||||||||||||||||||
|
Q.19 The 2009 Nobel prizes were awarded to work on
(A) human papilloma virus and ribosome
(B) Helicobacter pylori and human papilloma virus
(C) ribosome and telomerase
(D) telomerase and Helicobacter pylori
Q.20 Which of the following statements about yeast are CORRECT?
|
P. |
Yeast are fungi | ||
|
Q |
Yeast can form pseudohyphae | ||
|
R. |
Yeast reproduce asexually by budding | ||
|
S. |
Yeast are facultative anaerobes | ||
|
T. |
All yeast are pathogenic | ||
|
U. |
All yeast are dimorphic | ||
|
(A) |
P, Q, R and S |
(B) |
R, S, T and U |
|
(C) |
P, R, S and U |
(D) |
Q, R, S and T |
D
An L shaped wire PQR carrying a current i is placed at a distance s from the origin (see the figure below). The length PQ ts I such that I s, The magnitude of the magnetic field B at origin O is
|
(B) | 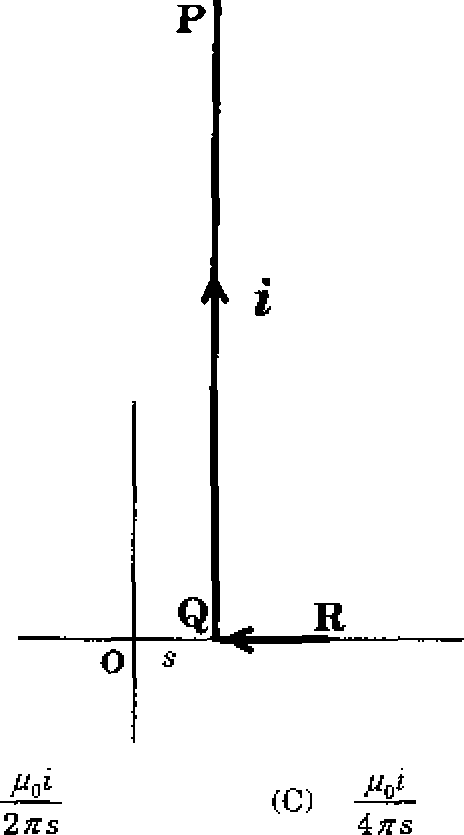 |
iV
JtS
W
%ns
{A)
(D)
The current (in A) in the given circuit, assuming the internal resistance of the batteries to be negligible, is
Q.22
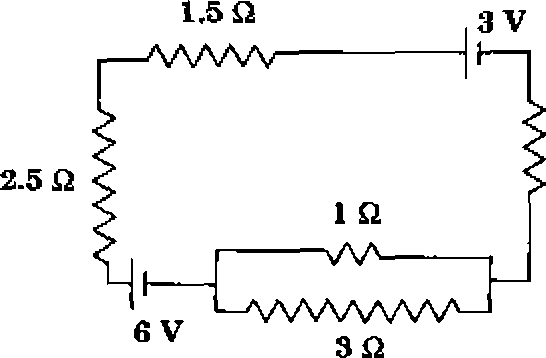 |
1.25 2 |
(B) i
2
Q.23
|
A gaseous mass M in the form of a thin disk of radius E is rotating with (t\ as shown in |
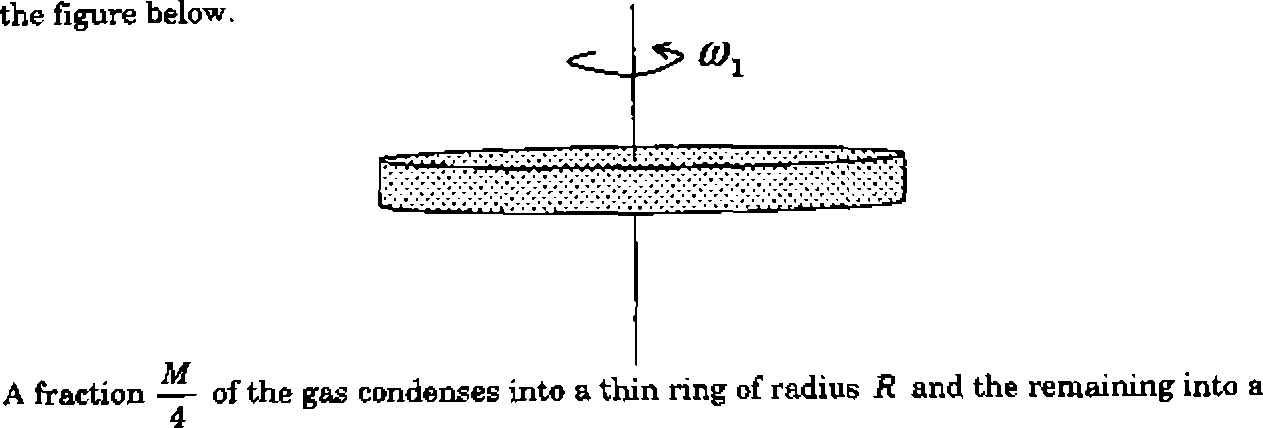 |
concentric disk of radius . The system now rotates with <3 about the same axis. The
2 ratio * is
The terminal speed (in m/s) of a vertically falling raindrop of radius 0.03 cm (g = 9.9 m/s2, ilair = xlO-4 poise and pwftt4r = 1000 kg/m3) is approximately
Q.24
<C) 1.10
(B) 0.55
(A) a.ii
(D) 2.20
In an RC circuit, a resistor of resistance 120 2 and a capacitor are connected to a 240 V, 50 Hz ac source. The circuit takes a current of 1.2 A. The reactance of the capacitor (in 2) is
Q.25
(A) 80
(B) 120
(D) 240
(C) 160
A bob of mass m of a long pendulum of length L is at a horizontal position P (see figure below). When released, it hits a ball of mass m placed at a position Q. Assume the collision to be elastic. After hitting the ball at Q, the bob will attain a height (with respect to Q) of
Q.26
Q.27 Signal recognition particles (SRPs) are
(A) protem-DNA complexes involved in protein sorting
(B) protein-RNA complexes involved in protein sorting
(C) protein-RNA complexes involved in RNA splicing
(D) protein-RNA complexes involved in cell cycle
Q.28 M phase promoting factor (MPF) facilitates cells to move from G2 to M phase during the cell cycle. The sudden decline in MPF at the end of the M phase is due to
(A) degradation of CDKs
(B) degradation of cyclins
(C) the reduced expression of cyclins
(D) an increase in the ratio of cell volume and genome
Q.29 Which of the following statements are TRUE regarding the dolichol phosphate pathway?
P, A 14-residue precursor oligosaccharide chain is synthesized in the ER
Q. A 14-residue precursor oligosaccharide chain is synthesized in the Golgi complex
R. It helps in N-linked glycosylation of proteins
S. It helps in O-linked glycosylation of proteins
(A) PandR (B) Q and R (C) Q and S (D) P and S
Q.30 Which one of the following is NOT TRUE about Klenow fragment?
(A) It is a proteolytic cleavage product of DNA polymerase I
(B) It has 5'*3' polymerase activity
(C) It has 3'-*5' exonuclease activity
(D) It has 5'*3' exonuclease activity
Q.31 In which one of the following options are the cellular compartments arranged in the increasing order of their pH?
(A) Nucleus, mitochondrial matrix, trans-Golgi network, lysosome
(B) Lysosome, nucleus, trans-Golgi network, mitochondrial matrix
(C) Lysosome, trans-Golgi network, nucleus, mitochondrial matrix
(D) Lysosome, nucleus, mitochondrial matrix, trans-Golgi network
|
Column I |
Column II |
|
P. Photoautotrophs |
1. Energy source: light Carbon source: C02 |
|
Q. Fhotoheterotrophs |
2. Energy source: light Principal carbon source: an organic compound |
|
R. Chemoautotrophs |
3. Energy source: chemical molecule Principal carbon source: COa |
|
S. Chemoheterotrophs |
4. Energy source: chemical molecule Principal carbon source*, an organic compound |
CA) P-l7 Q 2, R-3, S-4 (B) P-3, Q-2, R-4, S-l
(C) P-2> Q-i, R-l, S 3 CD) P-4, Q 3, R-2, S-l
Q.33 Which one of the following is NOT a saturated fatty acid?
(A) Palmitic acid (B) Stearic acid
(C) Oleic acid (D) Myristic acid
Q.34 The feeding relationship among the species in a community determine the community's
(A) secondary succession (B) ecological niche
(C) trophic structure (D) species richness
Q.35 Which one of the following techniques can be used to find whether a given sample contains glucose or galactose?
(A) Paper chromatography (B) Thin layer chromatography
(C) NMR spectroscopy (D) LTV spectroscopy
|
Q.36 Match the entries in Column I with those in Column II. | ||||||||
|
(A) P-1,Q-2,R'3 (B) P 2, Q-3, R-l (C) P-2, Q-l, R-3 (D) P 3,Q 2,R-1
Q.37 Which of the following statements pertaining to 2D gel electrophoresis of proteins is/are CORRECT?
P. While preparing the sample from a tissue, the sample should be dissolved in SDS Q. The duration for which the SDS gel is run should not vary to ensure reproducibility
(A) OnlyP (B) Only Q
(C) P and Q (D) Neither P nor Q
BT-8/24
An inductive coil of resistance 1 Q is connected to a 20 V battery. Neglect the internal resistance of the battery. The value of the induced emf (in V) in the coil at an instant when the current has risen to one-fourth of its steady value is
(A) 5 (B) 5V2 (C) 10 (D) 15
A charge +Q is uniformly distributed in a sphere of radius R. The magnitude of the electric field E at a distance from the center of the sphere is
Q.39
(A) 0 (B)
(C) --- (D) -
16 7ifcRz 8 xe0R'
A vibrating string of length i hag mass m. It vibrates with a fundamental frequency when stretched by a force of 1 N. This string will vibrate with seoond harmonic (i.e., first overtone) if the force is increased to
Q.40
(A) N (B) 42 N CO 4N <D) 8N
Electrons are emitted in photoelectric effect, and beta particles are emitted in radioactive decay of nuclei. Which one of the following statements is CORRECT?
Q.41
(A) The energy of photoelectrons is much greater than that of beta particles
(B) The energy of beta particles is much greater than that of photoelectrons
(C) The energies of photoelectrons and beta particles are of same order
(D) Beta particles and photoelectrons have different masses
Two sources of light are coherent if they emit radiation of
(A) unequal intensities, same wavelength and same phase
(B) equal intensity, same wavelength and different phases
(C) unequal intensities, same wavelength and different phases
(D) equal intensity, different wavelengths and different phases
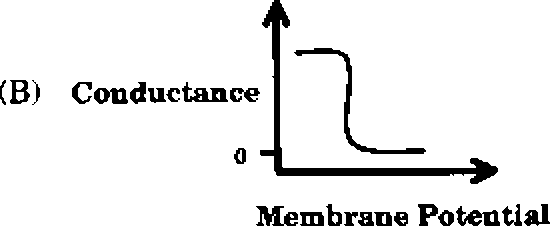
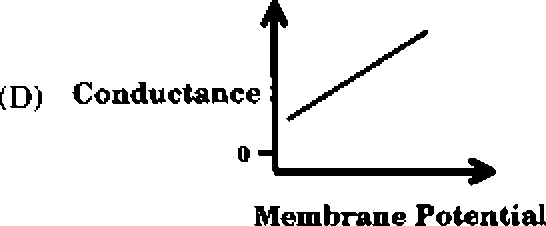
Q.43 Which one of the following schematics CORRECTLY depicts the variation of conductance as a function of membrane potential for the voltage-gated K+-channel?
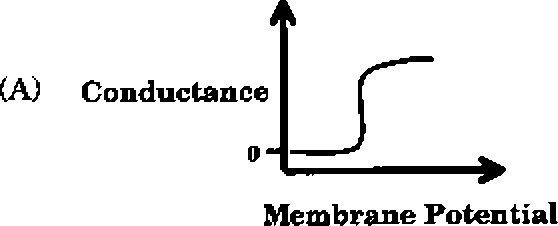
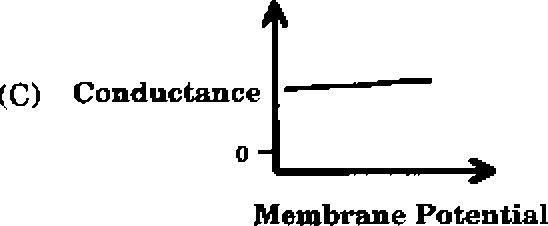
Q.44 In a xenogenic cell-based therapy, the donor and recipient belong to different species. In which of the following, do the donor and recipient belong to the same species?
|
p |
Autologous | ||
|
Q |
Allogenic | ||
|
R |
Syngeneic | ||
|
(A) |
Only P |
(B) |
Only P and Q |
|
(C) |
Only P and R |
(D) |
P, Q and R |
Q.45 According to the following schematic, which shows the effect of the ligand L on the allosteric protein P,
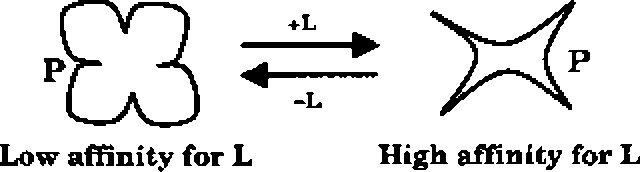
(A) L has to be an allosteric activator
(B) L has to be an allosteric inhibitor
(C) L can be either an allosteric activator or an allosteric inhibitor
(D) L is not an allosteric modulator of the protein
Q.46 Which one{s) among helicase, primase, telomerase and topoisomerase can form phosphodiester bonds?
(A) Only primase
(B) Primase and telomerase only
(C) Primase, telomerase and topoisomerase only
(D) All the four enzymes
Q.47 Type II hypersensitivity
(A) is antibody independent
(B) is complement independent
(C) is mediated by CDS* T cells
(D) involves antibody-mediated destruction of cells
Q.48 Which of the following statements relating to photosynthesis are CORRECT?
P. Carotenoids protect against toxic oxygen species
Q. When plants utilize blue light, they can harness more energy than when they utilize red light
R. The porphyrin ring in both chlorophyll and bacteriopheophytin has magnesium
S. Chemical modification of the porphyrin ring alters its absorption spectrum T. The Z-scheme, depicting the flow of electrons in photosynthesis, is based on oxidation potentials U. Carboxysomes are subcellular structures present in certain prokaryotes V. Efflux of magnesium from the thylakoid lumen into the stroma helps in the
activation of RuBisCo
(B) Q, R, S and V (D) P, S, T, U and V
(A) P, Q and R
(C) R, S, T and V
Q.49 Which of the following membranes have a proton-pumping ATPases?
BPM Bacterial plasma membrane
CIM Chloroplast inner membrane TGM Trans-Golgi membrane
LM Lysosomal membrane
MIM Mitochondrial inner membrane
VM Vacuolar membrane
|
(A) BPM, CIM, MIM and VM (C) CIM, LM, MIM and VM |
(B) BPM, TGM, MIM and VM (D) BPM, LM, MIM and VM |
(A) A mass m is enclosed in a spherical shell. The gravitational force on this mass due to another point mass M lying outside the shell is zero
(B) When an object of mass m is in motion under a gravitational force, both angular momentum and total mechanical energy are conserved
(C) The acceleration due to gravity decreases with increasing altitude
(D) The acceleration due to gravity is dependent on the mass of earth
An electromagnetic wave with a magnetic field vector
Q.51
B -200 x 10"9 T cos [(1.8 rad/m) y + {5.4 x 10s rad/s) t\ k
propagates along
|
(A) |
+ j |
and |
its |
electric |
field |
vector is |
along |
-i |
|
<B> |
~j |
and |
its |
electric |
field |
vector is |
along |
+ i |
|
(C) |
+ j |
and |
its |
electric |
field |
vector is |
along |
+ i |
|
CD) |
-j |
and |
its |
electric |
field |
vector is |
along |
A. -i |
Which one of the following statements is NOT CORRECT?
Q.52
(A) In an unbiased p-n junction, the electric potential of n-side is higher than that of the p-side
(B) When a p-n junction is forward biased, the width of the depletion region increases
(C) When a p-n junction is forward biased, the forward current is due to both electron and hole diffusion
CD) When a p-n junction is forward biased, the potential of the p-side increases
D
Q.53 Which one of the following statements is CORRECT for the reaction
2 HNOg (o<7) + Cu(s) + 2H4 (o) 2 N02 (g) + Cu2+ (aq) + 2 H20 {D?
(A) H+ is the oxidising agent, and Cu is the reducing agent
(B) H+ is the oxidizing agent, and HNOa is the reducing agent
(C) HNO3 is the oxidizing agent, and Cu is the reducing agent
(D) Cu is the oxidizing agent, and HN03 is the reducing agent
Q.54 Which of the following Fischer projections of glyceraldehyde have identical absolute configuration?
OH
HOH2C|H CHO
K
CHO
H
ch2oh
J
HO-
OH
M
H CHzOH
HO|CHOH H|CHO
CHO
L
(A) K, L and M (B) J, K and M
CC) J, KandL <D) J, L and M
Q.55 In a water solution, the concentration of OH at 25 C is lO-5 mole/liter. The concentration of H3Q* is
(A) 10-Ifi mole/liter (B) HT12 mole/liter
(C) lQr9 mole/liter (D) KT2 mole/liter
Q.56 The CORRECT order of basicity of the following amines P, Q, R and S is
H H
P Q R S
(AJS<P<Q<R (B) R < Q < P < S
(C) Q < P < R < S (D) P < Q < S < R
Q.57 The values of x and y in the given structure of Nylon 66 are
O O
{A) x = 4 and y = 6 (B) x - 6 and y = 4
(C) x = 6 and y ~ 6 (D) x = 4 and y = 4
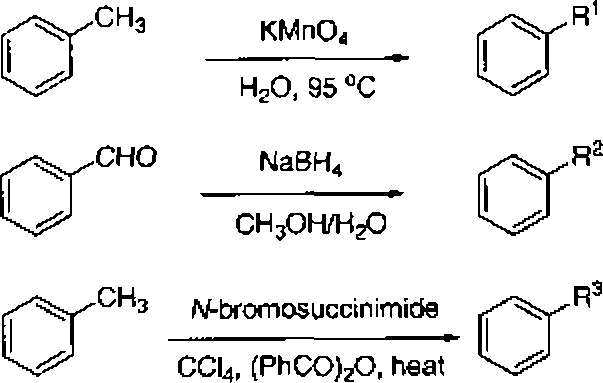
(A) R1 = CH2OH, R2 = CH*Br and Rs = COOH
{B) R1 = COOH, R2 = CH2Br and Ra = CH2OH
(C) R1 = COOH, R2 = CHOH and R3 = CH2Br
(D) R1 = CHiBr, R2 = COOH and R3 = CH2OH
The correct match between items of Column I and Column II is Column I P. Friedel-Crafts reaction Q. Baeyer-Villiger reaction R. Diels-Alder reaction
Q 59
Column n
Cycloaddition Walden inversion Oxidation
Aromatic electrophilic substitution
(B) P-4, Q-3, R-l, S-2 CD) P-4, Q-3, R 2, S I
1.
2*
3,
4.
S. Sn2 reaction
(A) P-3, Q-2, R 1, S 4
(C) P-l,Q-2,R-3,S-4
The molecular shape ofXeF2 is
Q 60
(B) trigonal pyramidal (D) linear
(A) trigonal bipyramidal
(C) V-shape
The number of signals in the NMR spectra of the following molecules P and Q, respectively, are
Q.61
| ||||||||||||
|
(B) 4 and 4 (C) 3 and 3 |
(D) 4 and 3
(A) 6 and 4
Q.62 Which of the following can be used as a biological weapon?
P. Bacillus anthracis
Q. Bacillus thuririgiensis
R. Bacillus subtilis
S. Ebola virus
(A) PandS (B) P and Q (C) P and E (D) Q and S
Q.63 The number of acetylated amino sugar(s) in the repeating unit of peptidoglycan is
Q.64 The genome of an adenovirus is a
(A) linear double stranded DNA (B) circular double stranded DNA
(C) plus-strand RNA (D) minus-strand RNA
Q.65 Which one of the following CANNOT be used to sterilize a heat-labile solution?
(A) Gamma radiation (B) Ethylene oxide
(C) Autoclaving (D) UV radiation
Q.66 Which one of the following second messengers targets protein kinase A?
(C) Diacylglycerol (D) Inositol 1,4,5-triphosphate
Q.67 Idiogram is
(A) a diagrammatic representation of karyotype of a species
(B) a diagrammatic representation of isotypic antibodies of a species
(C) a diagrammatic representation of the evolutionary tree of various species
(D) an autoradiogram profile of isotypic antibodies of a species obtained using l25l
Q.68 Spindle fibers, formed during the cell division, are composed of
(A) actin (B) collagen (C) myosin (D) tubulin
The differential equation representing the family of circles passing through the points (0, -6) and (0, b) is
(A) x2 -y2 +2xy& + b =0 (B) + y1 + 2xy- +fc2 = 0
dx dx
(C) x2 - y2-2xy- + b2 = 0 (D) x2 +y2 - + 62 - 0
dx dx
r a0*
The value of j is
Q.70
Q.71
Q.72
O 9 G>
K
(A) d~n (B) a* (C) -a (D) n
The point at which the tangent to the curve x3 + y3 = 6ry is parallel to y-axis (but is not y-axis) is
(A) (4, 22) (B) (23/2,42) (C) (22, SV4> (D> <24,22)
For p> 0, 9>0* if the maximum of px+qyt subject to the constraints 0<x, Oy, x + 2y<10 and 3x + y<15, exists at points (0,5) and (4,3), then the relationship between p and q is
(A) 2 p=q (B) p = 2q (C) p = 3q (D) 3 pq
The set of complex numbers z, which satisfy the equation |z-3|+-|z + 3| = 10 in the Argand plane, forms
Q.73
(A) a circle (B) a parabola (C) an ellipse (D) a hyperbola
If (x+iy) = u + l? , then + is
Q 74
Q.75
(A) 4(*2+y2) (B) 4(y2 -x2) (C) 4(x2-y2) (D) -4(*2+y2)
. 2
Saji and Milind are on a treasure hunt. The probability that Saji will find it is and
3
that both Saji and Milind will find it simultaneously is . The probability that Saji alone
finds it is
(A) - (B) - (C) - (D) I
4 2 4 6
Q.76 Considering the equation AG" = AH - TAS, which one of the following statements ia NOT CORRECT?
(A) When AG" is negative, the reaction is exergonic
(B) When AG " is negative, the reaction can occur spontaneously
(C) When AS is negative, the molecular disorder decreases during the reaction.
(D) When AH is negative, the reaction is endothermic
Q.77 The difference in the energies of the eclipsed and staggered conformations of ethane at 25C is approximately
(A) 5.40 kcal/mole <B) 2.70 kcal/mole
(C) 0.54 kcal/mole (D) 0 27 kcal/mole
Q.78 Which one of the following statements is NOT CORRECT?
(A) The second ionization potential of an atom is larger than its first ionization potential
(B) Atomic size increases from top to bottom in a group of the periodic table
(C) Electron affinity of an atom is the energy required to add an electron to its outermost orbit
(D) Electronegativity of an atom is its ability to attract electrons towards itself
Q.79 Name of the compound [Co(NH3)G]Cl3 is
(A) cobalt(III) hexaammine chloride (B) hexaamminecobalt chloride(III)
(C) hexaamminecobalt trichloride (D) hexaamminecobalt{I[[) chloride
Q.80 Which one of the following molecules has dipole moment?
(A) PC13 (B) BC13 <C) C02 (D) N2
Q.81 In a photochemical reaction, light is involved in
(A) initiation step only (B) propagation step only
(C) termination step only (D) propagation and termination steps
Q.82 Proteasomes are
(A) proteomes of lysosomes
(13) protein complexes which recognize and degrade ubiquitinated proteins
(C) protein and cholesterol complexes which help in cholesterol transport
(D) protein and RNA complexes which are involved in mRNA splicing
-1 0<x <1 2 1<x < 2 a: 2 <x <4
(A) limit exists but is not continuous at 1 (B) limit exists and is continuous at 1 {C} limit exists but is not continuous at 2 (D) limit exists and is continuous at 2
/(*) =
The minimum value of the function x3-6x2 + 9x + 10 in the interval [0, 4] is at
Q.84
Q.85
(A) 1 only (B) 1 and 3 (C) 0 and 3 (D) 3 only
The equation of the plane passing through the line of intersection of the planes 3x +2y = 5 and x + y + 2s+1 = 0, and containing the point (1, 2, 3) is
(B) 14# + 9y-2s = 26 (D) 9* + 14y + 2z = 43
(A) 14x-$y + 2z2 (C) 9x + 14y 2z = 31
If d and b are unit vectors inclined at an angle 0t then
d-b
Q.86
is
(C) 2 co
(D) 2 sin -2
(B) 2 sin 9
(A) 2 cos
1-2 0 2 3-1
-3 1 4
, the value of the determinant of adjoint of A is (B) 529 (C) 441 (D) 361
Q.87
For A =
(A) 576
The converse of the statement ux = 2 implies x2 = 4 * is
(A) x2 = 4 implies x = 2 (B) x2 = 4 implies * = -2
fC) =4 implies (r =2 or * = -2) (D) *2 =4 implies (x =2 and r =
The number of functions from the set {a, by ct d) to the set {1, 2, 31 is
(A) 12 (B) 36 <C) 64 (D) 81
Q.88
-2
Q.89
M is the set of natural numbers, I is the set of integers, US is the set of rational numbers, Ris the set of real numbers and C is the set of complex numbers. There is a bijection between
(B) 2 and R
(C) R and
(A) N and 1
Q.9I The removal of bursa of Fabricius from a chicken results in
(A) a delayed rejection of skin graft
(B) low serum levels of antibodies
(C) anemia
(D) a marked decrease in the number of circulating T lymphocytes Q.92 A mouse, which lacks thymus, is called
(A) SCLD mouse (B) NUDE mouse (C) BEIGE mouse (D) CBA/N mouse Q.93 Which one of the following is a gratuitous inducer of the lac operon?
|
(A) Galactose-(}{l,6)-glucoBe (C) O-Nitrophenylgalactoside |
(B) Galactose-0(l,4)-glucose <D) Isopropyl- (3-thiogalactoside |
Q.94 Which of the following statements pertaining to cell and tissue culture are CORRECT?
P. In a tissue culture incubator, increasing the partial pressure of C02 results in a decrease of the pH of the medium Q. An inactive telomerase is required for a cell to achieve immortality R. Antibiotics are added to the culture media to prevent microbial contamination
S. Hayflick limit refers to the number of cells that can grow in a culture flask T. Serum proteins are required for the adhesion of cells to the surface of a solid
substrate
(B) P, Q and T (D) Q, R and T
(A) P, Q, S and T (C) P, R and T
Q.95 In the schematic shown below v P, Q, R, S, T, U and V are metabolites.
P
|
Q . |
 |
|
The dotted lines denote (A) sequential feedback inhibition (C) repression |
(B) negative feedback inhibition (D) cumulative feedback inhibition |
The correct match between items of Column I and Column II is Column 1 Column II
P. Co2" (aq) 1- Colorless Q, Zn2+(aq) 2. Blue R. Cu2+ (aq) 3, Pink S, Niz+ (aq) 4. Green
(A) P-3, Q-l, R-2, S-4 (B) P-4T Q-2, R-l, S-3
<C) P-3, Q-4, R-2, S-l (D) P-2, Q-4, R-l, S-3
Considering the acidities of the given molecules, which one of the following orders is NOT CORRECT?
Q.97
Q.98
(A) CH4 < Nn2 < HzO < HF (B) SiH4 < FH3 < H2S < HC1
{C) HF < HC1 < HBr < HI (D) HzO < HS < HzTe < H2Se
Which one of the following alcohols undergoes dehydration with rearrangement involving a methyl migration?
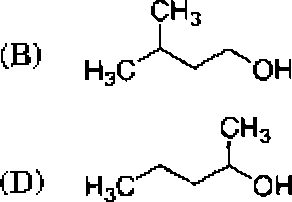
,A, HjCCH,
(C) H3C'/'OH
Which one of the following compounds gives acetone as one of the products when treated sequentially with (i) 03 and (ii) MejS?
Q.99
CH
2
ch3
(A) H3C>V-'CH3 (B)
(liters V 1
The units of rate constant (k) of a reaction are , If the order of the reaction
Q. 100
V mole ) s
is , the value of x is
(A) 0 (B) \ (C) 1 (D) |
A
End of the paper
BT-2Q/24
D
&
&
O
1V3S
o
S'
%
a
\
|
Attachment: |
| Earning: Approval pending. |
