Indian Institute of Technology New Delhi - (IIT) 2009 M.Sc BioTechnology Iit jam entrance for biotech - Question Paper
2009 - BT D
QUESTION BOOKLET CODE Test Paper Code : BT
|
Time : 3 Hours Max. Marks: 300 | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
MARKING SCHEME :
(a) For each correct answer, you will be awarded 3 (Three) marks.
(b) For each wrong answer, you will be awarded -1 (Negative one) mark.
(c) Multiple answers to a question will be treated as a wrong answer.
(d) For each un-attempted question, you will be awarded 0 (Zero) mark.
|
Name | |||||||
|
Roll Number | |||||||
(A) CH3-CH2-COOH (B) h2c=chcooh
(C) HOC-COOH (D) CH3-CH2-CH2-OOH
Q.2 Which of the following compounds, upon mass spectral analysis, give a base peak at m/z 119?
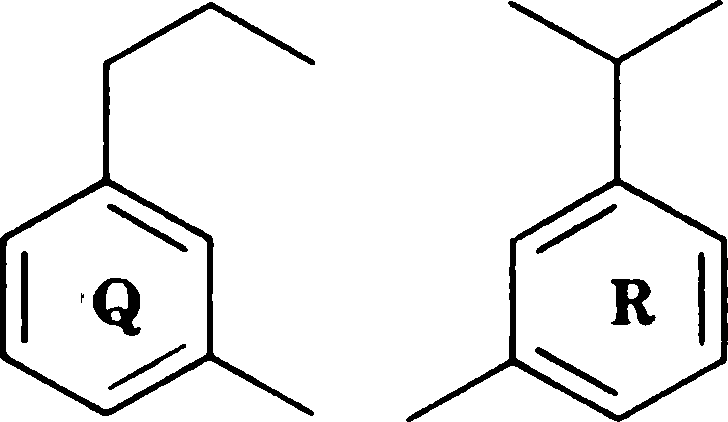 |
|
(C) Q and R |
(A) P, Q and R (B) P and Q
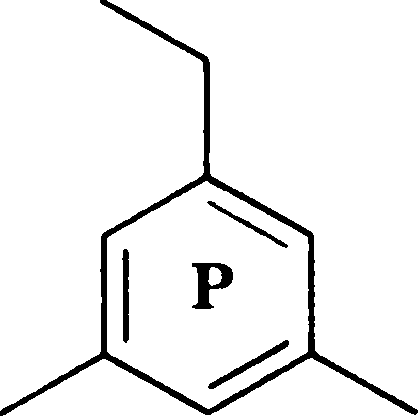
(D) P and R
Q.3 The order of melting point of LiF, LiCl, LiBr and Lil is
|
(A) LiF * LiCl < LiBr > Lil (C) LiF > LiCl - LiBr < Lil |
(B) LiF < LiCl < LiBr < Lil (D) LiF > LiCl > LiBr > Lil |
Q.4 Jahn-Teller distortion is a common phenomenon for octahedral complexes with
(A) a high spin d5 configuration (B) a d9 configuration
(C) a low spin d6 configuration (D) ad3 configuration
Q.5 The spin only magnetic moment of [CoFg]3- is
(A) 0.0 BM (B) 1.73 BM (C) 2.40 BM (D) 4.90 BM
Q.6 XeFg is isoelectronic and isostructural with
(A) PF5 (B) IF5 (C) PtF5 (D) C1F5
Q.7 Despite a large difference in their atomic number, Zr(40) and Hf(72) have comparable atomic radii. This is because
(A) both elements are in the same period of the periodic table
(B) of the lanthanide contraction
(C) of the actinide contraction
(D) of the presence of half-filled /-orbitals in Hf
Q.8 Let x and y be two numbers such that 0 < x < 1 and 0 < y < 1. Then the sum of the infinite series jc(s + y) + *2 (*2 + y2) + xz (x3 + ya) +......+ xn (xn + yn) +... is
xy
(B)
(D)
1 -xy xy 1 -xy
1-xz a:2
<c> r +
1 X
(A)
1 -y2 1 -xy y2 xy
1 -y2 1 -xy
xy
Q.9 Let z = 2 + i4s represent the vertex of a square inscribed in the circle |z-l| = 2. Then one of the adjacent vertices of the square is
(A) S-l + i (B) 1-S+i (C) l+V3 + i (D) \-S-i
Q.10 The area of the triangle formed by the lines joining the vertex of the parabola x2 =8 y to the ends of the latus rectum is
(A) 8
(B) 4
(C) 16
(D) 2
Q.ll The co-ordinates of a point, where the line y = x + 42 touches the circle x2 + y2 = 1, are
f I /ii>\ f i i>\ /'iiN
fj_
42 * 42
2___i
42.' 42)
1 1
42'42.
(A)
(B)
(C)
(D)
42' 42
dy
Q.12 Let y(x) be the solution of the differential equation x = x + y, xe (0, <) satisfying
dx
the initial condition y (1) = 0. Then, as x 0,
(B) y(x)~* 1
(D) y(x) does not have a limit
Q.13 The problem of minimizing the function 2x + 3y subject to the constraints x + y = 5, x<,2y y<i 4, oc>0 and yO has
(A) multiple solutions (C) no solution
(B) an optimal solution (D) an unbounded solution
Q.14 The co-ordinates of the point, where the line joining the points A = (l, 2, 3) and B = (2, 3, 4) crosses the ary-plane, are
Q.15 Electron affinity of nitrogen is
(A) higher than that of phosphorus (B) lower than that of phosphorus
(C) comparable to that of phosphorous (D) identical to that of phosphorus
Q.16 Kevlar(-[-NHC6H4-NHCO-C6H4-CO-]n-),polyethene(-[-CH2-CH2-]-), Dacron(-[-0-
CH2-CH2-0-C0-C6H4-C0-]n-) and Lexan(-[-C0-0-C6H4-C(CH3)2-C6H4-0-]n-) represent
(A) a polyamide, a polyolefin, a polyester and a polycarbonate respectively
(B) a polyester, a polyolefin, a polyamide and a polycarbonate respectively
(C) a polyamide, a polyolefin, a polycarbonate and a polyester respectively
(D) a polyamide, a polyolefin, a polyester and a polyester respectively
Q.17 Plasmid A and plasmid B were digested with BamHI and analyzed by agarose gel electrophoresis. If Plasmid A gave two fragments and plasmid B gave three fragments, then which of the following inferences are CORRECT?
P. Plasmid A has three sites and is circular
Q. Plasmid B has three sites and is circular
R. Plasmid A has two sites and is linear
S. Plasmid B has two sites and is linear
(A) P and Q (B) Q and R (C) P and S (D) RandS
Q.18 Cholera toxin manifests its action by
P. the ADP-ribolysation of Gj protein
Q. the transfer of ADP-ribose from NAD+ to the Gg protein
R. the inhibition of phosphodiesterase S. the activation of adenylate cyclase
(A) PandR (B) P and S (C) Q and S (D) Q and R
Q.19 When cultured in vitro with a suitable combination of growth regulators, plant parenchyma and collenchyma cells become meristematic. This phenomenon is called
(A) differentiation (B) maturation
(C) apoptosis (D) dedifferentiation
Q.20 Which one of the following statements about sieve tube elements in plants is NOT CORRECT?
(A) They are supported by companion cells
(B) They must die to become functional
(C) They link end to end forming sieve tubes
(D) They translocate organic nutrients
Q.21 IM + B + C= then cot cot fi+cot C
2 cot A cot B cot C
(A) -1
(B) 1
(C) 0
(D) 2
Q.22 If a two digit number k is 4 times the sum of its digits and 2 times the product of its digits, then the number is
(A) 36
(B) 48
(C) 20
(D) 45
Q.23 Two bicycles start off to a slow race with initial velocities 4 m/s and 2 m/s and uniform accelerations 1 m/s2 and 2 m/s2, respectively. If both of them cover the same distance in the same time, then the distance covered is
(A) 12 m
(B) 16 m
(C) 24 m
(D) 36 m
Q.24 If the curve y= 5xs+ bx2 +cx+5 touches the x-axis at the point (1, 0), then the pair (6, c) is
(A) (5,5)
(B) (-5,5)
(C) (5, -5)
(D) (-5,-5)
Q.25 The value of f-8m* C8* dx is
*1 + sin* cos*
(A) 1 (B) 0
(C) -1
(D) 2
'i+y'2 vi-yj
Q.26 If 0 < y < 1, then the coefficient of yn in the expansion of (A) 4 n (B) 1 (C) n-1
is
(D) 2 n
_! ( 11-sinx V1 + sin x
Q.27 If 0 <> x < /r/2, then the value of tan
is
n-x
(O f-*
(D) - -4 2
(A)
Which one of the following is NOT part of a molluscan body plan?
(A) Mantle (B) Radula (C) Visceral mass (D) Trachea
A clade is
Q.29
Q.30
Q.31
Q.32
Q.33
(A) a type of phylogenetic tree
(B) a group of evolutionarily related species sharing a common ancestor
(C) an extinct species
(D) a tool for constructing a phylogenetic tree Kupffer cells are found in
(A) stomach (B) liver
(C) small intestine (D) large intestine
Ecological succession refers to
(A) changes in community composition after a disturbance
(B) the process by which a species become abundant
(C) the building of soil nutrients
(D) changes in a forest as trees grow taller
During fertilization, the movement of the pollen tube towards the ovule is guided by a protein released from
|
(A) Egg |
(B) |
Synergid |
|
(C) Antipodal cell |
(D) |
Polar nuclei |
|
Choose the correct matches. | ||
|
Disease |
Glands/organs | |
|
P. Mumps |
1. |
Pancreas |
|
Q. Colitis |
2. |
Stomach |
|
R. Hepatitis |
3. |
Salivary gland |
|
S. Gastritis |
4. |
Large intestine |
|
5. |
Liver | |
|
(A) P-4, Q-l, R-2, S-5 |
(B) |
P-3, Q-4, R-5, S-l |
|
(C) P-3, Q-4, R-5, S-2 |
(D) |
P-2, Q-3, R-l, S-5 |
The correct sequence for sperm migration after its production in testis is
(A) Seminiferous tubule epididymis > vas deferens urethra
(B) Urethra vas deferens epididymis * seminiferous tubule
(C) Epididymis > vas deferens urethra seminiferous tubule
(D) Seminiferous tubule * vas deferens epididymis * urethra
R
Q.35 Statement 1 : Isotopes are chemically identical
Statement 2: Chemical reactions do not depend on the number of neutrons in the atom Which of the following is CORRECT?
(A) Statements 1 and 2 are correct and 2 is the correct explanation for 1
(B) Statements 1 and 2 are correct, but 2 is not the correct explanation for 1
(C) Statement 1 is correct, but statement 2 is wrong
(D) Statement 1 is wrong, but statement 2 is correct
Q.36 The wavelength of electromagnetic radiation emitted by the hydrogen atom in the first excited state is given as X. The wavelength corresponding to the transition from the third excited state to the ground state is
<B> 7* 4
(C)
(D) A 32
Q.37 N0 atoms of a certain radioactive element undergo radioactive decay. After 100 days the
N
number is reduced to . The mean life of the element, in days, is
100
ln(2)
(B)
(C) 200
(D) 100
(A) 100 ln(2)
Q.38 A point particle of mass M moving with a constant speed u collides with another point particle of mass 2M, which is at rest. After the collision, if the second particle moves with a non-zero speed, the speed of the first particle is
2 u
(A)
(B)
(C) 0
(D) **
2
Q.39 Two satellites of masses M and 2M are orbiting around the earth in circular orbits of radii R and 2R, respectively. The ratio of their speeds is
(A) 2 a/2 : 1 (B) V2 : 1 (C) 1 :4 (D) 2:1
Q.40 A particle is projected vertically upwards from the surface of earth with an initial speed of 40 m/s. The acceleration of the particle when it reaches the maximum height is
The phenomenon of expression of only one allele of an immunoglobulin gene in lymphocytes is known as
(A) allelic exclusion (B) allelic inclusion
(C) allelic variation (D) allelic heterogeneity
The antibody class that can pass from the mother to the fetus in humans is (A) IgA (B) IgD (C) IgG (D) IgM
Q.42
Q.43
Q.44
Q.45
Q.46
Q.47
Which of the following statements is NOT CORRECT about MHC class II proteins?
(A) They are recognized by CD4 co-receptors
(B) They are composed of a and p chains
(C) They are involved in presenting antigen to helper T cells
(D) They are present in the T cell cytoplasm
Bilirubin is formed due to the degradation of
(A) erythrocytes (B) leucocytes (C) hepatocytes (D) macrophages
|
Match the diseases in Group I with the corresponding hormones in Group II. | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
Which of the following covalent bond types are found in the structure of ATP?
(A) N-glycoside, thioester, phosphomonoester
(B) phosphoanhydride, phosphomonoester, N-glycoside
(C) ester, ether, phosphoanhydride
(D) ether, thioester, phosphomonoester
Let F = i+ j be the force acting at the point P = (1, 0, 0), where i and j are the unit vectors along the ac-axis and y-axis, respectively. Then the moment of F about the line through origin in the direction of j is
(A) ~ (B) (C) -1 (D) 0
Let f(x) - min {V*, x2, x3} and a be the area bounded by the curve y-f (jc), the x-axis
Q.50
Q.51
Q.52
Q.53
xe [0. 4]
and the ordinates at x = 0 and x = 4. Then the value of a is
(A) 16/3 (B) 64/3 (C) 59/12 (D) 64
An urn contains 3 red, 5 black and 7 yellow balls. If a ball is drawn at random, then the probability that the ball drawn is not yellow is
(A) 7/15 (B) 8/15 (C) 7/8 (D) 1/7
Let A be a non-singular square matrix of order 3. If B is the matrix obtained from A by adding 3-multiple of its first row to its second row, then the value of det(2i4-1fi) is
(A) 8 (B) 3 (C) 6 (D) 2
The pressure difference between the inside and the outside of a liquid drop is
(A) linearly proportional to the radius of the drop
(B) inversely proportional to the radius of the drop
(C) proportional to the square of the radius of the drop
(D) zero
Q.54
In a Youngs double slit experiment using light of wavelength X, the interference pattern of fringe width 2.5 mm is observed. If the same set up is used with a light of wavelength 2X, the fringe width would be
(A) 1.25 mm (B) 2.5 mm (C) 5 mm (D) 10 mm
A ray of light traveling through a medium of refractive index 42 is incident on an interface with another medium of refractive index 1, at an angle of incidence of 30. Which of the following statements is CORRECT?
(A) The ray undergoes total internal reflection
(B) The ray is fully transmitted
(C) The ray just grazes the interface
(D) The ray is partly reflected and partly transmitted
Q.56 Choose the correct set of matches between the function and the corresponding cellular structure.
|
Function |
Structure | ||
|
P. |
Protein synthesis |
1. |
Lysosomes |
|
Q. |
Intracellular digestion |
2. |
Ribosomes |
|
R. |
Protein secretion |
3. |
Microtubules |
|
S. |
Macromolecular traffic |
4. |
Mitochondria |
|
5. |
Golgi apparatus | ||
|
(A) |
P-l, Q-2, R-5, S-3 |
(B) |
P-2, Q-l, R-4, S-5 |
|
(C) |
P-3, Q-5, R-l, S-4 |
(D) |
P-2, Q-l, R-5, S-3 |
Q.57 2, 4-Dinitrophenol inhibits mitochondrial function by
(A) inhibiting ATP synthesis (B) inhibiting electron flow
(C) dissipating the electrochemical gradient (D) decreasing oxygen permeability
Q.58 The premature termination of polypeptide synthesis due to a stop codon can be overcome by a compensatory mutation in tRNA. This genetic phenomenon is referred to as
(A) intragenic suppression (B) extragenic suppression
(C) codon bias (D) true reversion
Q.59 Myoglobin shows a hyperbolic response, while hemoglobin shows a sigmoidal response for oxygen binding. Which of the following statements are TRUE with respect to this observation?
P. Hemoglobin binds 2, 3-BPG while Myoglobin does not
Q. Hemoglobin exists in two different conformational states while Myoglobin does not R. Hemoglobin is a tetramer while Myoglobin is a monomer S. Hemoglobin is present in RBCs while Myoglobin is present in the muscle
(A) R and S (B) S and P (C) P and Q (D) Q and R
Q.60 An increase in enzyme activity in a cell is mechanistically due to transcription. This mechanism can be demonstrated by
(A) measuring total enzyme activity in the cell free extract
(B) ELISA
(C) Northern blot
(D) Western blot
Q.61 Galactosemia is a recessive single gene genetic disorder, caused due to the mutation in any one of the three genes involved in galactose catabolism. A family consists of 10 normal children with both parents suffering from galactosemia. This is most likely because of
(A) epistasis (B) reversion
(C) suppression (D) complementation
Q.62 If R is the resistance and C is the capacitance in an electric circuit, the dimensions of RC are the same as that of
(A) Current
(B) Voltage
(C) Charge
(D) Time
Q.63 One mole of an ideal mono-atomic gas of initial volume is compressed adiabatically to V
a volume . If the initial temperature is T0, then the final temperature is
|
0.67 |
1.67
0.67
(C) r0(2)
(D) T0( 2)
Q.64 The internal energy of 3 moles of an ideal mono-atomic gas at absolute temperature T is given by
(B) fkRT
(A) -RT 2
(C) -RT 2
Q.65 A real gas behaves as an ideal gas at (A) constant volume (C) low density
(B) constant pressure (D) high pressure
Q.66 Treating the diol shown below with strong acid gives compound E.
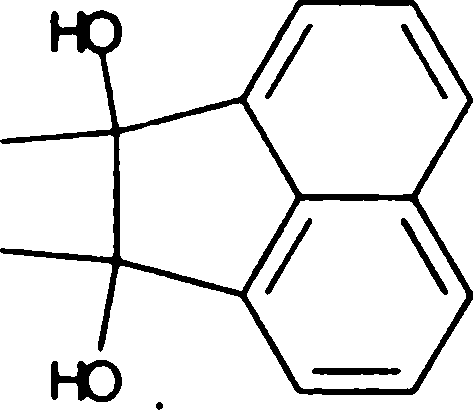
Compound E displays a prominent absorption band at 1710 cm-1 in its IR spectrum. The most likely structure of E is
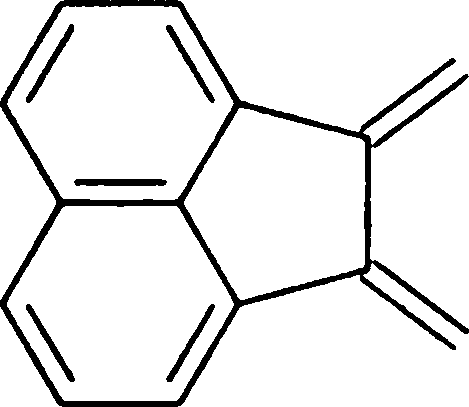
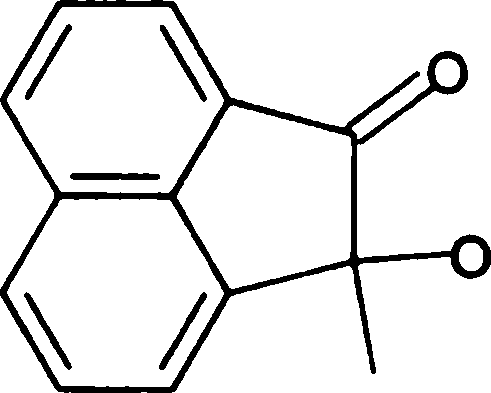 |
H |
(A)
(B)
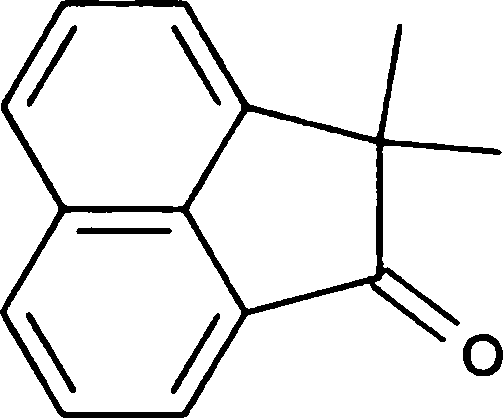
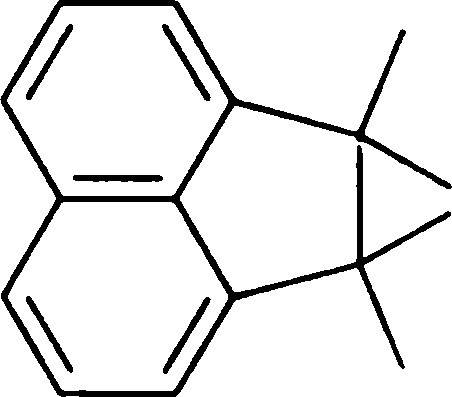
(C)
(D)
Q.67 Okazaki fragments are formed during DNA synthesis, because '
P. DNA synthesis extends from 5 to 3 direction
Q. their synthesis are opposite to the direction of replication fork
R. DNA ahead of replication fork is positively supercoiled
S. DNA synthesis is semi-conservative
(A) Q and R (B) P and Q (C) RandS (D) S and P
Q.68 Which of the following elements must be present in a plasmid cloning vector?
P. Origin of replication
Q. Selectable marker
R. Unique restriction sites
S. Promoter element upstream of the unique restriction site
(A) S and P (B) Q and R (C) RandS (D) P and Q
Q.69 The metabolite pair that is NOT formed directly from pyruvate is
(A) Oxaloacetate and acetaldehyde (B) Alanine and ethanol
(C) Acetyl CoA and alanine (D) Lactate and oxaloacetate
Q.70 Which of the following pairs of protocols is used before ligating two DNA molecules with incompatible 5 overhangs?
P. Filling in with a Klenow fragment
Q. Si nuclease digestion
R. Dephosphorylation of 5 phosphate
S. Phosphorylation of 3 hydroxyl group
(A) P and Q (B) Q and R (C) R and S (D) S and P
Q.71 Which of the following amino acids is a major precursor of one-carbon units?
(A) Proline (B) Alanine (C) Serine (D) Methionine
Q.72 A mutation in the operator, which prevents the binding of the repressor resulting in the constitutive expression of the lac operon, is referred to as
(A) semi-dominant (B) trans-dominant (C) co-dominant (D) cis-dominant
Q.73 Two double stranded DNA samples that are identical with respect to the number of base pairs, but differ significantly in their GC content, can be separated by
(A) Density gradient centrifugation (B) Agarose gel electrophoresis
(C) Dialysis (D) Oligo-dT column chromatography
Q.67 Okazaki fragments are formed during DNA synthesis, because '
P. DNA synthesis extends from 5 to 3' direction
Q. their synthesis are opposite to the direction of replication fork
R. DNA ahead of replication fork is positively supercoiled
S. DNA synthesis is semi-conservative
(A) Q and R (B) P and Q (C) RandS (D) S and P
Q.68 Which of the following elements must be present in a plasmid cloning vector?
P. Origin of replication
Q. Selectable marker
R. Unique restriction sites
S. Promoter element upstream of the unique restriction site
(A) S and P (B) Q and R (C) R and S (D) P and Q
Q.69 The metabolite pair that is NOT formed directly from pyruvate is
(A) Oxaloacetate and acetaldehyde (B) Alanine and ethanol
(C) Acetyl CoA and alanine (D) Lactate and oxaloacetate
Q.70 Which of the following pairs of protocols is used before ligating two DNA molecules with incompatible 5 overhangs?
P. Filling in with a Klenow fragment
Q. Si nuclease digestion
R. Dephosphorylation of 5 phosphate
S. Phosphorylation of 3 hydroxyl group
(A) P and Q (B) Q and R (C) RandS (D) S and P
Q.71 Which of the following amino acids is a major precursor of one-carbon units?
(A) Proline (B) Alanine (C) Serine (D) Methionine
Q.72 A mutation in the operator, which prevents the binding of the repressor resulting in the constitutive expression of the lac operon, is referred to as
(A) semi-dominant (B) Jrcms-dominant (C) co-dominant (D) cis-dominant
Q.73 Two double stranded DNA samples that are identical with respect to the number of base pairs, but differ significantly in their GC content, can be separated by
(A) Density gradient centrifugation (B) Agarose gel electrophoresis
(C) Dialysis (D) Oligo-dT column chromatography
Q.74 Match the test from Column 1 with the sample pairs in Column 2 so that positive identification is possible.
|
Column 1 |
Column 2 | ||
|
(J) |
Flame test |
(1) |
CH3-CO-CH3 and CH3-CO-C6H5 |
|
(K) |
Tollens reagent test |
(2) |
H-CO-H and CH3-CO-C2H5 |
|
(L) |
Water solubility test |
(3) |
Glucose and Starch |
|
(M) |
Iodoform test |
(4) |
CH3-CO-C6H6 and C6H5-CO-C6H5 |
|
(5) |
NaCl and Nal | ||
|
(6) |
CH3NH-CO-NHCH3 and CH3-CO-NH |
(A) J-4, K-3, L-2 and M-l (B) J-4, K-4, L-5 and M-l
(C) J-l, K-2, L-3 and M-4 (D) J-3, K-5, L-6 and M-4
Q.75 Under neutral conditions, O-glycosides, unlike the free sugars from which they are derived, do not exhibit mutarotation. This is because
(A) the anomeric hydroxyl group exists as an acetal
(B) the anomeric carbon atom exists in the open chain form
(C) the anomeric carbon atom exists as a hemi-acetal
(D) O-glycosides exist as a racemic mixture and hence mutarotation cannot be observed
Q.76 The reaction CaC03(s) = CaO(s) + C02(g) is not favored at 298 K. Given that for this reaction at 298 K, A..H0 = 200 kJmol'1 and AJS0 = 200 JK"1mor1, the lowest temperature at which this reaction will proceed in the forward direction is
(A) 801 K (B) 901 K (C) 1001 K (D) 1101 K
Q.77 The graphs P, Q and R show the variation of rate constant (k) with temperature. The reactions represented by P, Q and R, respectively, are
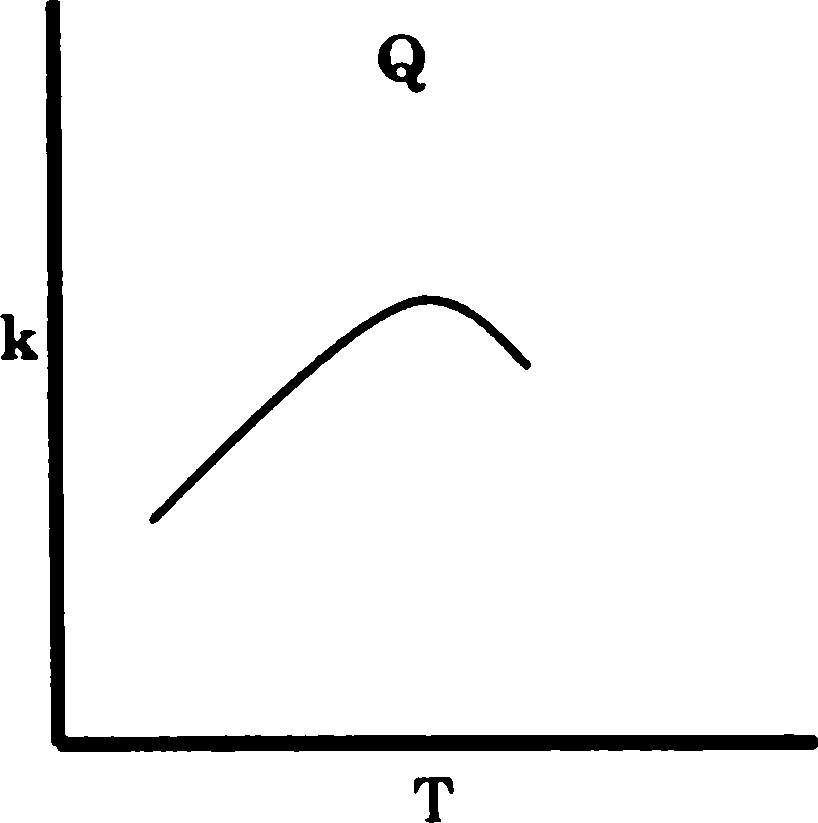
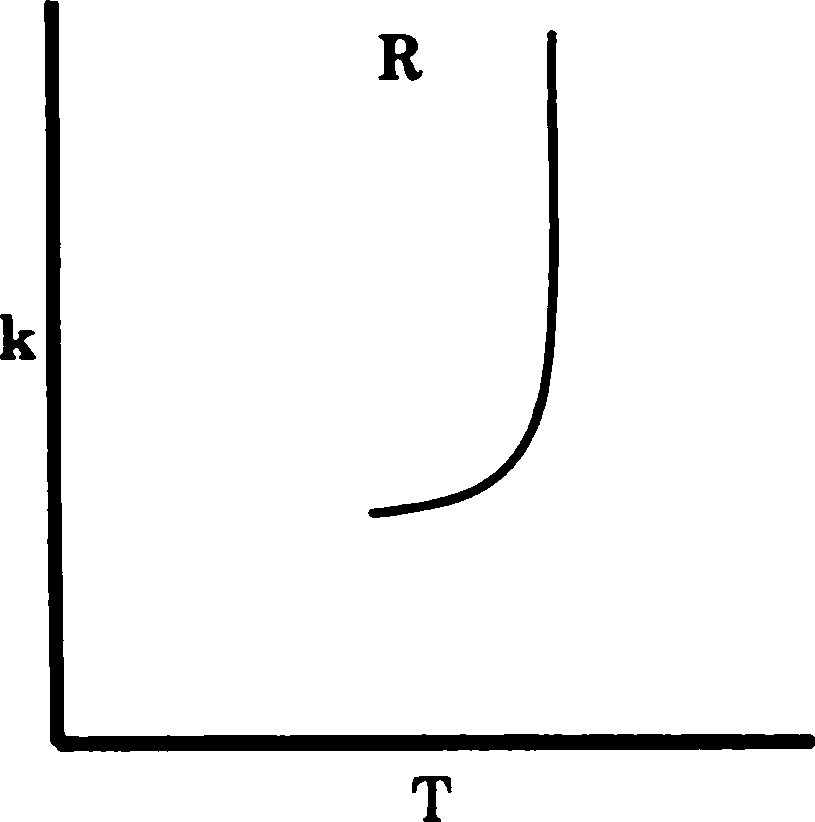
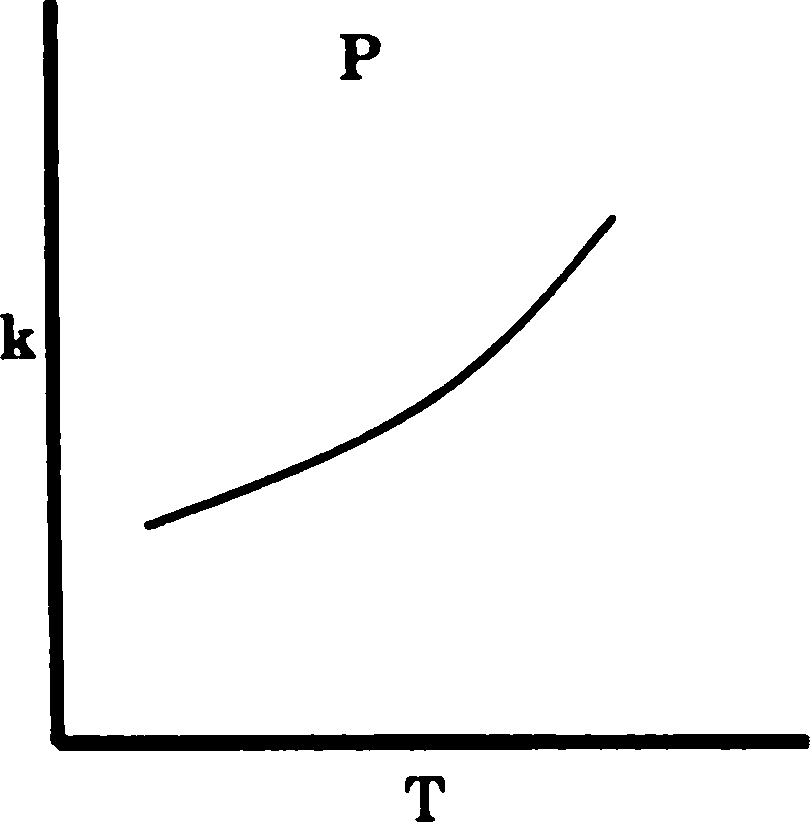
(A) P - Arrhenius type, Q - an enzyme catalysed and R - a chain reaction
(B) P - an enzyme catalysed, Q - Arrhenius type and R - a chain reaction
(C) P - Arrhenius type, Q - a chain reaction and R - an enzyme catalysed reaction
(D) P - a chain reaction, Q - an enzyme catalysed and R - Arrhenius type reaction
Q.78 Which of the following pairs of amino acids have two chiral carbons in their structure?
(A) Thr and lie (B) Tyr and Trp (C) His and Met (D) Leu and Gly
Q.79 Base pairing between inosine and uridine occurs through
(A) Hoogsteen base pairing (B) Watson-Crick base pairing
(C) Wobble base pairing (D) Purine-purine base pairing
Q.80 The net charge on a protein will be negative when the pH is
(A) at its isoelectric pH (B) above its isoelectric pH
(C) below its isoelectric pH (D) at neutral pH
Q.81 The molecule that functions as a natural thiol reductant in a cell is
(A) Glutathione (B) Methionine (C) Dithiothreitol (D) Cystine
Q.82 The two pathways required for the net synthesis of glucose from triglycerides in germinating groundnut seeds are
(A) Hexose monophosphate shunt and Gluconeogenesis
(B) Calvin cycle and Glyoxalate cycle
(C) Glycolysis and Cori cycle
(D) Glyoxalate cycle and Gluconeogenesis
Q.83 Match the antibiotic with its inhibitory mode of action.
|
Antibiotic |
Mode of action | ||
|
P. |
Penicillin |
1. |
Protein synthesis |
|
Q. |
Rifamycin |
2. |
Protein glycosylation |
|
R. |
Tunicamycin |
3. |
RNA polymerase |
|
S. |
Sulfanilamide |
4. |
Folate biosynthesis |
|
5. |
Peptidoglycan synthesis | ||
|
(A) |
P-5, Q-3, R-l, S-2 |
(B) |
P-3, Q-4, R-2, S-l |
|
(C) |
P-2, Q-l, R-3, S-4 |
(D) |
P-5, Q-3, R-2, S-4 |
Q.84 Which of the following statements are TRUE for a mutation that changes the codon from UAC to UAG?
P. It is a nonsense mutation Q. It is a missense mutation
R. It is a point mutation S. It is a transversion
Q.85 The product M obtained in the substitution reaction shown below is
2-
/C1 Pt-Cl
Cl
+ 2 NH,
M
/
Cl
NH,
Cl
/
Pt-Cl
/
(A)
H3N
(B)
Pt-NH,
H3N
/
/
Cl
Cl
NH<
NH,
(D)
(C)
Pt
aci
H3N*
H3N NH, NH3
Q.86 The order of X-N-X bond angle in NH3, NF3 and NC13 is
(A) NH3<NC13<NF3 (B) NC13>NH3>NF3
(C) NH3>NC13>NF3 (D) NC13>NF3>NH3
Q.87 The deep pink color of aqueous KMnC>4 solution is due to
(A) a very strong d - d transition
(B) a very weak d - d transition
(C) a strong ligand to metal charge transfer interaction
(D) a strong metal to ligand charge transfer interaction
The concept of Spontaneous generation of life was disproved by experiments using swan neck flasks. This experiment was conducted by
(D) Lister
(B) Pasteur
(A) Koch
(C) Schwann
Consider the following three groups and choose the correct match.
Q.89
|
Vitamin Cofactor PI. Riboflavin Ql. TPP P2. Thiamine Q2. CoA P3. Nicotinamide Q3. Biocytin P4. Pantothenate Q4. NADP P5. Cobalamine Q5. FAD P6. Biotin |
Enzyme Rl. Pyruvate carboxylase R2. Succinate dehydrogenase R3. Glucose 6-phosphate dehydrogenase R4. Pyruvate decarboxylase R5. Succinate thiokinase R6. Hexokinase |
(A) P1-Q5-R2, P2-Q1-R4, P3-Q4-R3, P4-Q2-R5, P6-Q3-R1
(B) P1-Q5-R2, P4-Q1-R6, P3-Q4-R3, P4-Q2-R5, P6-Q3-R1
(C) P1-Q5-R2, P2-Q1-R4, P1-Q4-R6, P4-Q2-R5, P6-Q3-R1
(D) P1-Q5-R2, P2-Q1-R4, P3-Q4-R3, P5-Q2-R6, P6-Q3-R1
For a reversible reaction S P, the equilibrium concentration of P is 100 times that of S. The equilibrium constant for this reaction in the presence of an enzyme catalyst will be
Q.90
Q.91
(A) 0.01 (B) 1 (C) 100 (D) 1000
Which of the following statements are TRUE with respect to membrane fluidity of a phospholipid bilayer?
P. Increasing proportion of long chain fatty acids decreases membrane fluidity Q. Increase in cholesterol content increases membrane fluidity R. Increasing proportion of cis unsaturated fatty acids increases membrane fluidity
S. Increasing proportion of trans unsaturated fatty acids increases membrane fluidity
(A) P and Q (B) P and R (C) R and S (D) Q and S
Choose the correct set of words denoted by (P), (Q) and (R) for the following statement. Pheophytin is a (P) molecule in which the central atom (Q) has been replaced by two atoms of (R).
(A) P - plastocyanin, Q - copper, R - hydrogen
(B) P - plastocyanin, Q - zinc, R - oxygen
(C) P - chlorophyll, Q - magnesium, R - oxygen
(D) P - chlorophyll, Q - magnesium, R - hydrogen
Q.93 A wheel of radius R rolls on a horizontal surface without slipping. If the centre of mass moves with a speed v, the instantaneous speed at the highest point on the wheel is
(A) v/2 (B) 0 (C) v (D) 2u
Q.94 A quantity Z = XY is to be estimated by measuring X and Y. If the absolute errors in the measurement of X and Y are AX and AY, respectively, then the absolute error in Z is
(A) AZ = YAX + XAY (B) AZ = AXAY (C) AZ = ta + *Y (D) AZ=j+'
Q.95 Which of the following is NOT CORRECT for electromagnetic waves propagating in vacuum?
(A) Electromagnetic waves with different wavelengths travel with the same speed
(B) The electric and the magnetic fields are perpendicular to each other and perpendicular to the direction of propagation
(C) The magnetic field is along the direction of propagation
(D) Electromagnetic waves carry energy
Q.96 A charged particle, with an initial velocity in the xy plane, is subjected to a uniform magnetic field along the 2-axis. Which of the following is the CORRECT statement?
(A) The particle will experience a force along the e-axis
(B) The speed of the particle remains constant
(C) Acceleration of the particle is zero
(D) The particle moves in a helical path
Q.97 A uniform electric field E0i, where i is the unit vector along the *-axis, exists in a region. A cube of side a'is kept with one of its corners coinciding with the origin and three edges along the x, y, z axes. Which one of the following is the CORRECT statement?
(A) The total charge inside the cube is non zero
(B) The flux of electric field through each face is zero
(C) The flux of electric field through all the faces are equal, but non zero
(D) The net flux of electric field through all the faces is zero
Q.98 The greater efficiency of detergents over soaps as cleaning agents in aqueous media is best described by
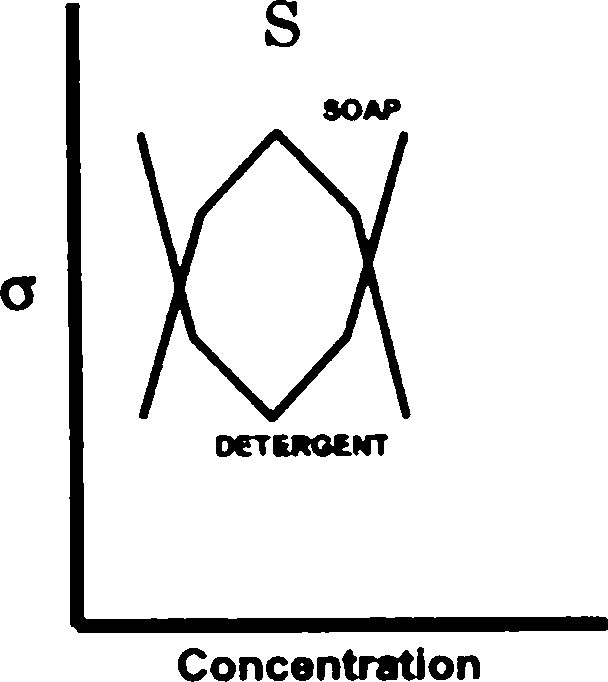
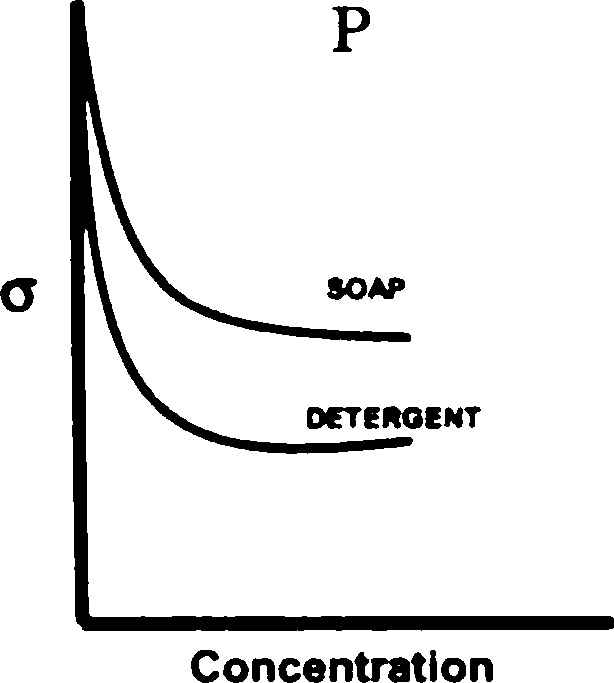
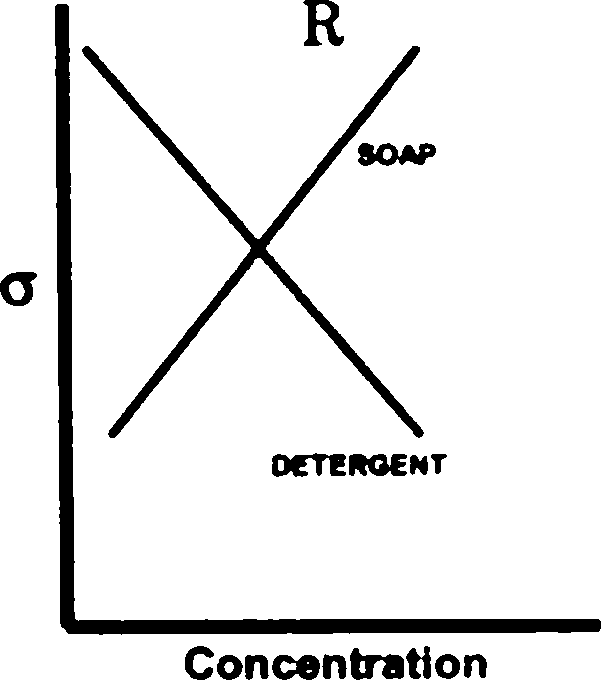
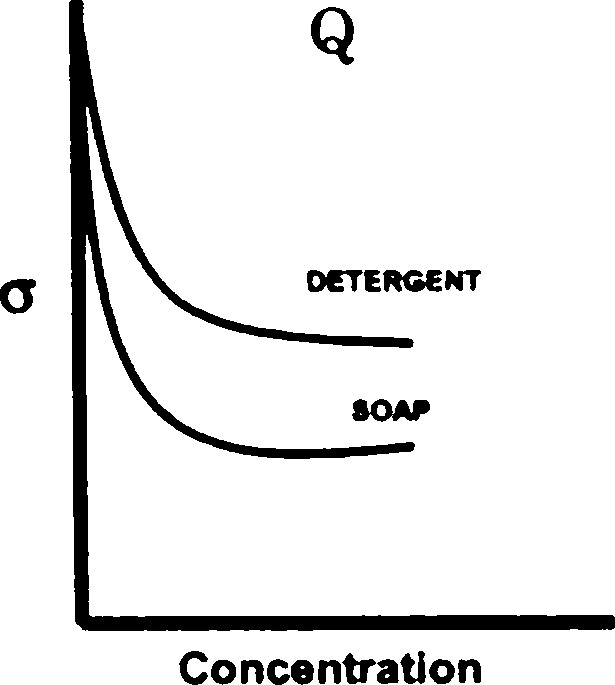
[ where a is the surface tension of water ]
(A) graph S (B) graph R (C) graph Q (D) graph P
|
Q.99 The pairs of structures P, Q and R are shown as Fischer projections. P, Q and R, respectively, represent |
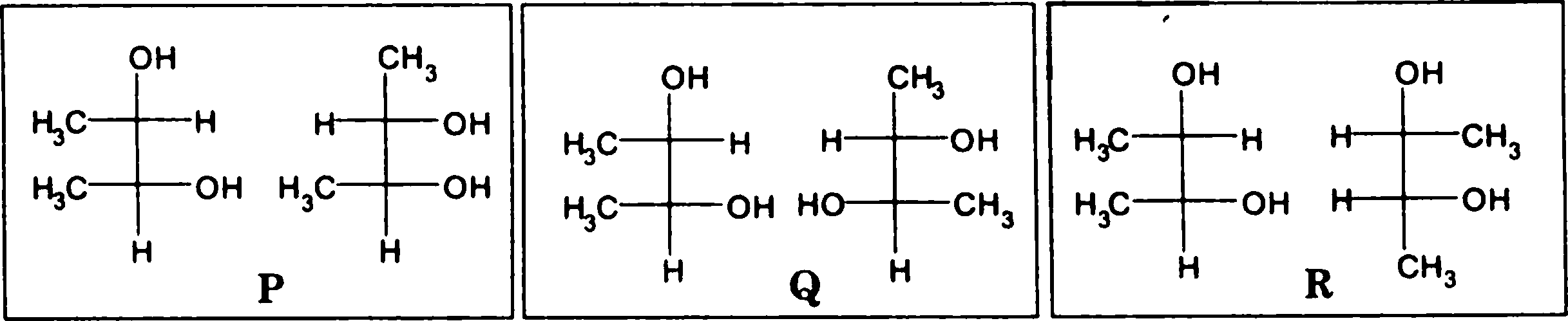 |
(A) the same molecules, diastereomers and enantiomers
(B) the same molecules, enantiomers and diastereomers
(C) the same molecules, the same molecules and enantiomers
(D) enantiomers, diastereomers and enantiomers
Q.100 The product obtained by the nitration, at low temperature, of meta xylene is
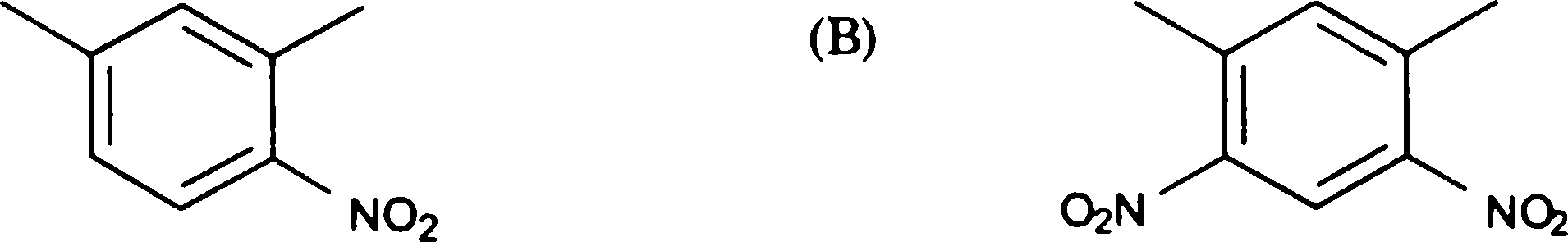
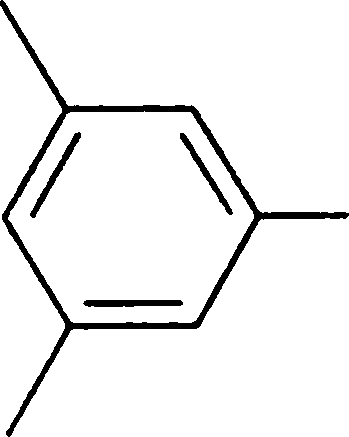
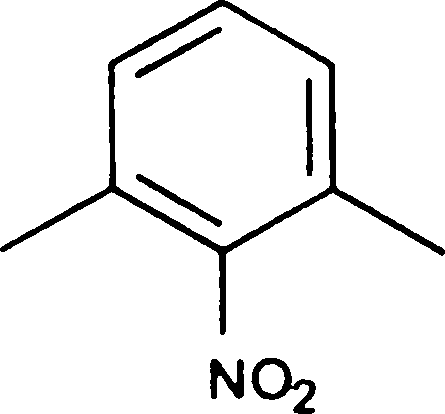
(C)
(D)
no2
BT-17/20
|
Attachment: |
| Earning: Approval pending. |
