Indian Institute of Technology New Delhi - (IIT) 2011 M.Tech Computer and Information Science Computer science and information technology engineering gate- - Question Paper
2011 CH
CH : CHEMICAL ENGINEERING
Duration: Three Hours Maximum Marks: 100
Read the following instructions carefully.
1. Write your name and registration number in the space provided at the bottom of this page.
2. Take out the Optical Response Sheet (ORS) from this Question Booklet without breaking the seal.
3. Do not open the seal of the Question Booklet until you are asked to do so by the invigilator.
4. Write your registration number, your name and name of the examination centre at the specified locations on the right half of the ORS. Also, using HB pencil, darken the appropriate bubble under each digit of your registration number and the letters corresponding to your test paper code (CH).
5. This Question Booklet contains 24 pages including blank pages for rough work. After opening the seal at the specified time, please check all pages and report discrepancy, if any.
6. There are a total of 65 questions carrying 100 marks. All these questions are of objective type. Questions must be answered on the left hand side of the ORS by darkening the appropriate bubble (marked A, B, C, D) using HB pencil against the question number. For each question darken the bubble of the correct answer. In case you wish to change an answer, erase the old answer completely. More than one answer bubbled against a question will be treated as an incorrect response.
7. Questions Q. 1 - Q.25 carry 1-mark each, and questions Q.26 - Q.55 carry 2-marks each.
8. Questions Q.48 - Q.51 (2 pairs) are common data questions and question pairs (Q.52, Q.53) and (Q.54, Q.55) are linked answer questions. The answer to the second question of the linked answer questions depends on the answer to the first question of the pair. If the first question in the linked pair is wrongly answered or is unattempted, then the answer to the second question in the pair will not be evaluated.
9. Questions Q.56 - Q.65 belong to General Aptitude (GA). Questions Q.56 - Q.60 carry 1-mark each, and questions Q.61 - Q.65 carry 2-marks each. The GA questions begin on a fresh page starting from page 19.
10. Unattempted questions will result in zero mark and wrong answers will result in NEGATIVE marks. For Q.1 - Q.25 and Q.56 - Q.60, A mark will be deducted for each wrong answer. For Q.26 - Q.51 and Q.61 - Q.65, 2A mark will be deducted for each wrong answer. The question pairs (Q.52, Q.53), and (Q.54, Q.55) are questions with linked answers. There will be negative marks only for wrong answer to the first question of the linked answer question pair, i.e. for Q.52 and Q.54, % mark will be deducted for each wrong answer. There is no negative marking for Q.53 and Q.55.
11. Calculator is allowed whereas charts, graph sheets or tables are NOT allowed in the examination hall.
12. Rough work can be done on the question paper itself. Additionally, blank pages are provided at the end of the question paper for rough work.
|
Name | ||||||||
|
Registration Number |
CH | |||||||
_CT
Q. 25 carry one mark each.
2011
Q.l
Q.l
Match the polymerisation mechanisms in Group I with the corresponding polymers in Group II
P. Chain growth/addition polymerisation I. Polyethylene
Q. Step growth/condensation polymerisation II. Polyvinyl chloride
111. Polyethylene terephthalate
(A) P - III; Q-UI (B) P -1,II; Q - III
(C) P -11,111; Q -1 (D) P -1; Q-IIJII
Which ONE of the following sequences is arranged according to INCREASING calorific value?
Q-2
(A) Producer gas, Natural gas, Water gas (B) Natural gas, Producer gas, Water gas
(C) Producer gas, Water gas, Natural gas (D) Water gas, Natural gas, Producer gas
The CORRECT sequence of process equipment used in the production of sulphuric acid from sulphur by contact process is
Q-3
(A) burner, catalytic converter, 98% sulphuric acid absorption tower, oleum absorption column
(B) catalytic converter, oleum absorption column, 98% sulphuric acid absorption tower, burner
(C) burner, catalytic converter, oleum absorption column, 98% sulphuric acid absorption tower
(D) burner, oleum absorption column, catalytic converter, 98% sulphuric acid absorption tower Hydrotreating is used for
Q-4
(A) removal of water from crude oil
(B) treatment of crude oil with water
(C) improving octane number of gasoline
(D) removal of sulphur and nitrogen from petroleum fractions Zeolite ZSM-5 is added to commercial FCC catalyst for
(A) promoting S02 reduction
(B) promoting CO oxidation
(C) improving tolerance to metal content in feed
(D) enhancing Octane number
Q.6 Minimum input required to calculate the blank diameter for a torispherical head is
(A) crown radius
(B) crown radius, knuckle radius and length of straight flange
(C) knuckle radius and length of straight flange
(D) crown radius and knuckle radius
Q.7 Match the process parameters in Group I with the measuring instruments in Group II GROUP I GROUP II
P. Flame temperature Q. Composition of LPG R. Liquid air temperature
I. Thermocouple
II. Radiation pyrometer
III. Gas chromatograph
(A) P - III, Q - I, R - II (C) P - II, Q - III, R -1
(B) P -1, Q - III, R - II (D) P - II, Q -1, R - III
Q.8 The range of standard current signal in process instruments is 4 to 20 mA. Which ONE of the following is the reason for choosing the minimum signal as 4 mA instead of 0 mA?
(A) To minimise resistive heating in instruments
(B) To distinguish between signal failure and minimum signal condition
(C) To ensure a smaller difference between maximum and minimum signal
(D) To ensure compatibility with other instruments
q 9 Minimum work (W ) required to separate a binary gas mixture at a temperature T0 and pressure P0 is
' h ' fpure,2
/i
/pure, 1
+ y2ln
yi In
where v, and y2 are mole fractions, f , and fpiire2 are fugacities of pure species at T0 and P0,
and /] and f2 are fugacities of species in the mixture at T0, P0 and y, . If the mixture is ideal then W is
(A) 0 (B) -RT0[y{ In y{ + y2 In y2]
(D) RT0
Q. 10 R is a closed planar region as shown by the shaded area in the figure below. Its boundary C consists of the circles Ci and C2-
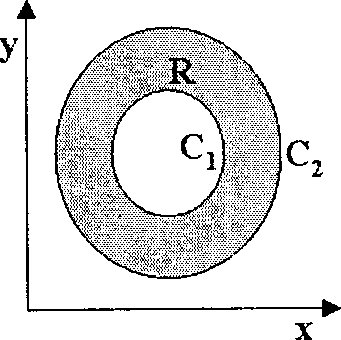
dp'
If Fx{x, y),F2{x,y), L and - are all continuous everywhere in R, Greens theorem states
dy dx
f 3 E1 77
that IT ---L dxdy = l(/71 dx + F2 dy). Which ONE of the following alternatives
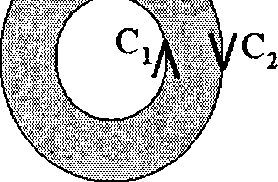
CORRECTLY depicts the direction of integration along C?
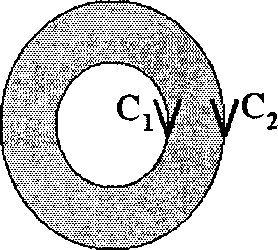
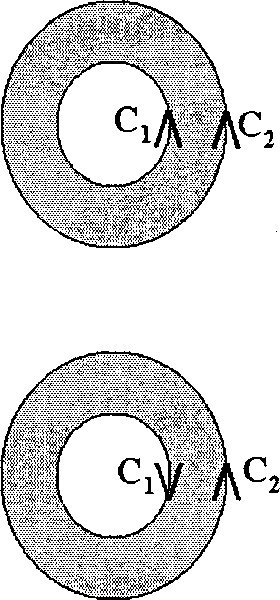
(B)
(D)
(A)
(C)
Q. 11 Which ONE of the following functions y (x) has the slope of its tangent equal to
ax
Note: a and b are real constants
x2 +b
x + b
(C) y = .
(A) y = -
(B) y = ax + b
a
(D) y - 'jajp' + b
Q.12 Let /lj=-land A3 be the eigenvalues and =
|
be the corresponding |
eigenvectors of a real 2x2 matrix R . Given that P = V2 j, which ONE of the following
matrices represents P RP?
|
(A) |
|
Q.13 The partial molar enthalpies of mixing (in J/mol) for benzene (component 1) and cyclohexane (component 2) at .300 K and 1 bar are given by AHl = 3600*2 and A//2 = 3600.x:,2 , where x,
and x2 are the mole fractions. When ONE mole of benzene is added to TWO moles of cyclohexane, the enthalpy change (in J) is
(A)3600 (B)2400 (C)2000 (D) 800
Q.14 One mole of methane is contained in a leak proof pistoncylinder assembly at 8 bar and 1000 K. The gas undergoes isothermal expansion to 4 bar under reversible conditions. Methane can be considered as an ideal gas under these conditions. The value of universal gas constant is 8.314
Jmol-1 K~'. The heat transferred (in kJ) during the process is
(A) 11.52 (B) 5.76 (C)4.15 (D) 2.38
Q.15 Consider the following two cases of movement of particles. In Case I, the particle moves along the positive y-direction and in Case II, the particle moves along negative y-direction. Gravity acts along the positive y-direction. Which ONE of the following options corresponds to the CORRECT directions of buoyancy acting on the particles?
|
y ! * |
i |
y i |
|
i3 |
i |
(A) Positive y-direction for both the cases
(B) Negative y-direction for Case I, positive y-direction for Case II
(C) Negative y-direction for both the cases
(D) Positive y-direction for Case I, negative y-direction for Case II
Q. 16 Match the pumps in Group I with the corresponding fluids in Group II.
P. Gear pump I. Highly viscous liquid
Q. Peristaltic pump II- Aqueous sterile liquid
III. Slurry
(A) P - III, Q - I (B) P - II, Q - I
(C)P-III, Q-II (D) P -1, Q - II
Q.17 Consider two black bodies with surfaces Si (area = 1 m2) and S2 (area = 4 m2). They exchange heat only by radiation. 40% of the energy emitted by Si is received by S2. The fraction of energy emitted by S2 that is received by Si is
(A) 0.05 (B) 0.1 (C) 0.4 (D) 0.6
Q. 18 In film type condensation over a vertical tube, local heat transfer coefficient is
(A) inversely proportional to local film thickness
(B) directly proportional to local film thickness
(C) equal to local film thickness
(D) independent of local film thickness
Q.19 Ammonia (component 1) is evaporating from a partially filled bottle into surrounding air (component 2). The liquid level in the bottle and the concentration of ammonia at the top of the bottle are maintained constant. /V, is the molar flux relative to a fixed location in space and J, is the molar flux with respect to the average molar velocity of the constituent species in the gas phase. Assume that air in the bottle is stagnant. Which ONE of the following is CORRECT?
(A) Ai = constant, N2 = 0, Ji + J2 = 0 (B) jVi + N2 = 0, Jx + J2 = 0
(C) Ni+ N2 = 0, = constant, J2 = 0 (D) /V, = constant, N2 = 0, J\ - constant, J2 = 0
Q.20 Simultaneous heat and mass transfer is occurring in a fluid flowing over a flat plate. The flow is laminar. The concentration boundary layer will COINCIDE with the thermal boundary layer, when
(A) Sc = Nu (B) Sh = Nu (C) Sh = Pr (D) Sc = Pr
Consider an irreversible, solid catalysed, liquid phase first order reaction. The diffusion and the reaction resistances are comparable. The overall rate constant (ka) is related to the overall mass transfer coefficient (km) and the reaction rate constant (k) as
Q.21
Reactant R forms three products X, Y and Z irreversibly, as shown below.
Q.22
X
The reaction rates are given by rx =kxCR, rY = kYClR5 and rz =kzCR. The activation energies
for formation of X, Y and Z are 40, 40 and 5 kJ/mol respectively. The pre-exponential factors for all reactions are nearly same. The desired conditions for MAXIMIZING the yield of X are
(A) high temperature, high concentration of R (B) high temperature, low concentration of R
(C) low temperature, high concentration of R (D) low temperature, low concentration of R
In an orifice meter, if the pressure drop across the orifice is overestimated by 5%, then the PERCENTAGE error in the measured flow rate is
Q.23
Q.24
(A) +2.47 (B) +5 (C) -2.47 (D) -5
Two systems are available for compressing 6 m3/hr of ambient air to 10 bar. The first one uses a single stage compressor (Kl) and the second one uses a multistage compressor with inter-stage cooling (K2). Which ONE of the following statements is INCORRECT?
(A) K2 will have knockout pots in between the stages
(B) Discharge temperature of Kl will be higher than that of K2
(C) K2 will consume more power than Kl
(D) Cost of K2 will be more than that of Kl
In a thin-walled cylindrical vessel of thickness t with inside radius r, the internal gauge pressure is p. The hoop stress and the longitudinal stress in the shell are 0/( and CT/ respectively. Which ONE of the following statements is TRUE?
Q.25
pr pr pr pr
(A)oA=-,oz=- (B) ah= ,Oi=
pr pr pr pr
(C)ofc=-,az = T (D )ah=T,al=-
Q. 26 to Q. 55 carry two marks each.
Q.26 Unit vectors in x and z directions are and k respectively. Which ONE of the following is the directional derivative of the function F(x, z) = In |x2 + z.2 j at the point P: (4,0), in the direction of
(A) hr ; fO 1 lDl
2V2 <B> i (C) 1 2V2
Q.27 Which ONE of the following choices is a solution of the differential equation given below?
dx x x x
Note: c is a real constant
/ w c-x2 c + 2x2
(A)>'=Wy=-r
c + 2x c-x
(r-\ C_A;3 C + 2x3
(O y=-7 (D) y=-T
c + 2x c x
7 dx
Q.28 The value of the improper integral j --r- is
(A) -In (B) 0 (C) n (D) 271
Q.29 Fuel cell stacks are made of NINE membrane electrode assemblies (MEAs) interleaved between TEN bipolar plates (BPs) as illustrated below. The width of a membrane electrode assembly and a bipolar plate are normally distributed with MmeA = MEA =0-01 and jUgp=5, 0BP = 0-1 respectively. The widths of the different layers are independent of each other.
|
Stack width | |
 |
{Not drawn to scale} |
 |
|
*1 I* ME A width BP width |
Which ONE of the following represents the CORRECT values of ( stack stack ) or tie
overall fuel cell stack width?
(B) (51.35, 1.09)
(C) (5.15,0.10)
(D) (5.15,0.11)
(A) (51.35,0.32)
Q.30 In the fixed point iteration method for solving equations of the form x = g(x), the (+!)* iteration value is xn+i = g(x), where xn represents the nlh iteration value. g(x) and corresponding initial guess value x0 in the domain of interest are shown in the following choices. Which ONE of these choices leads to a converged solution for x ?
|
(A) (B) |
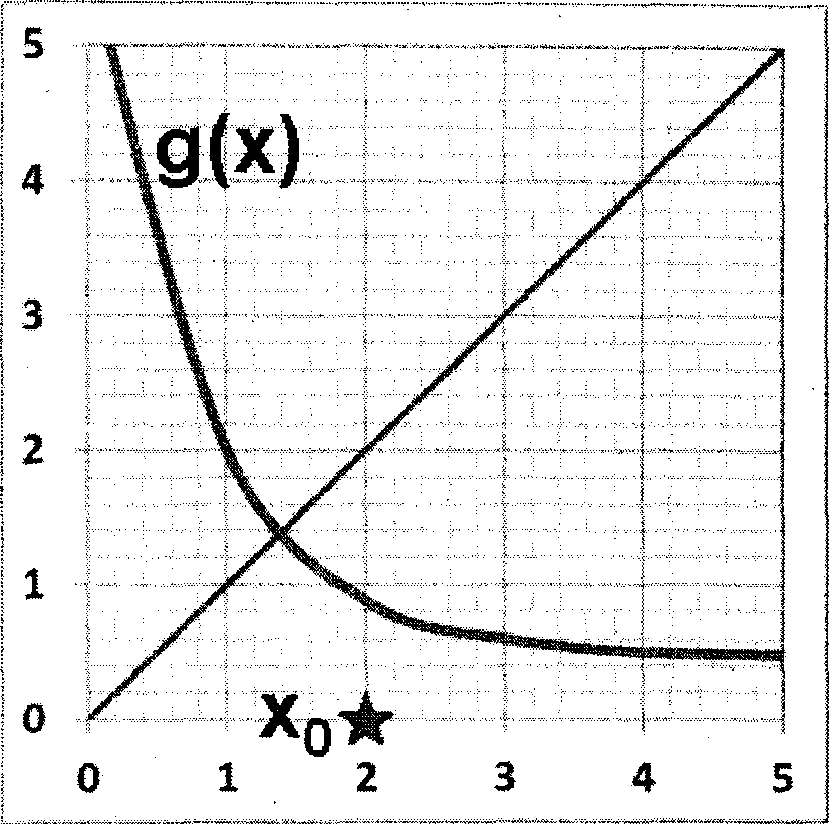 |
(C) (D)
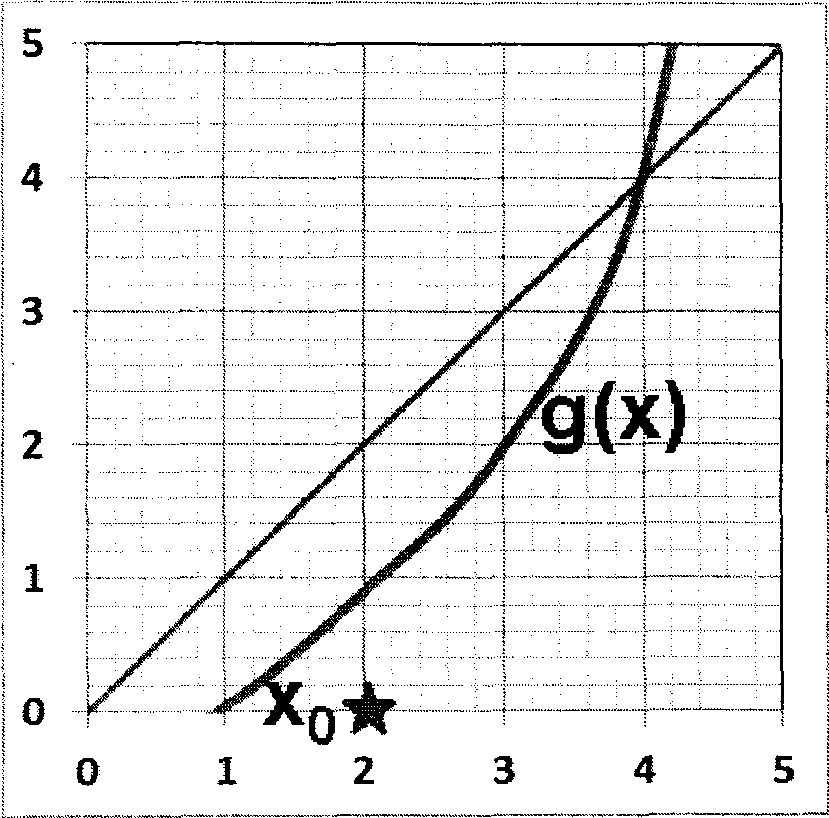
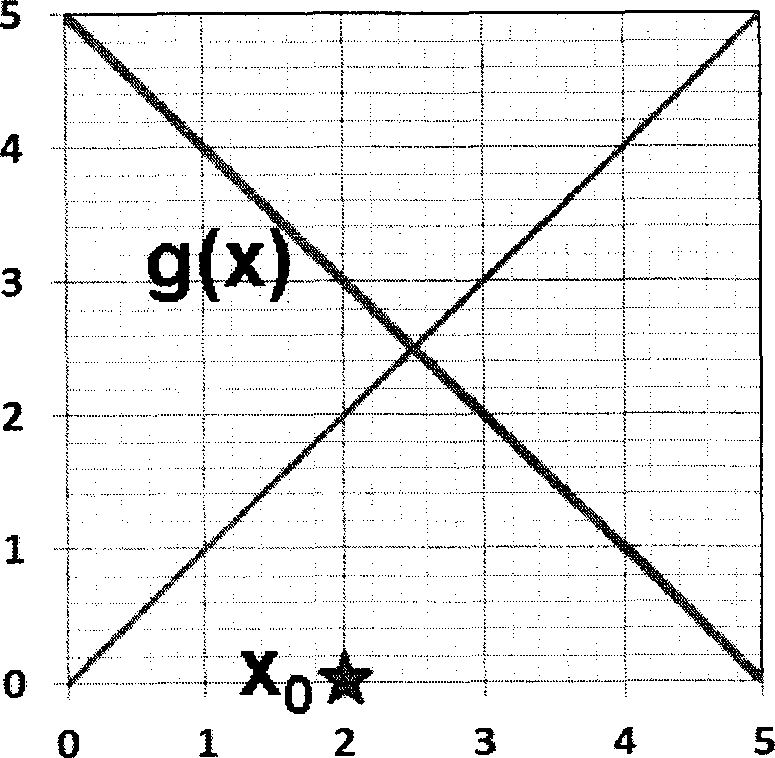
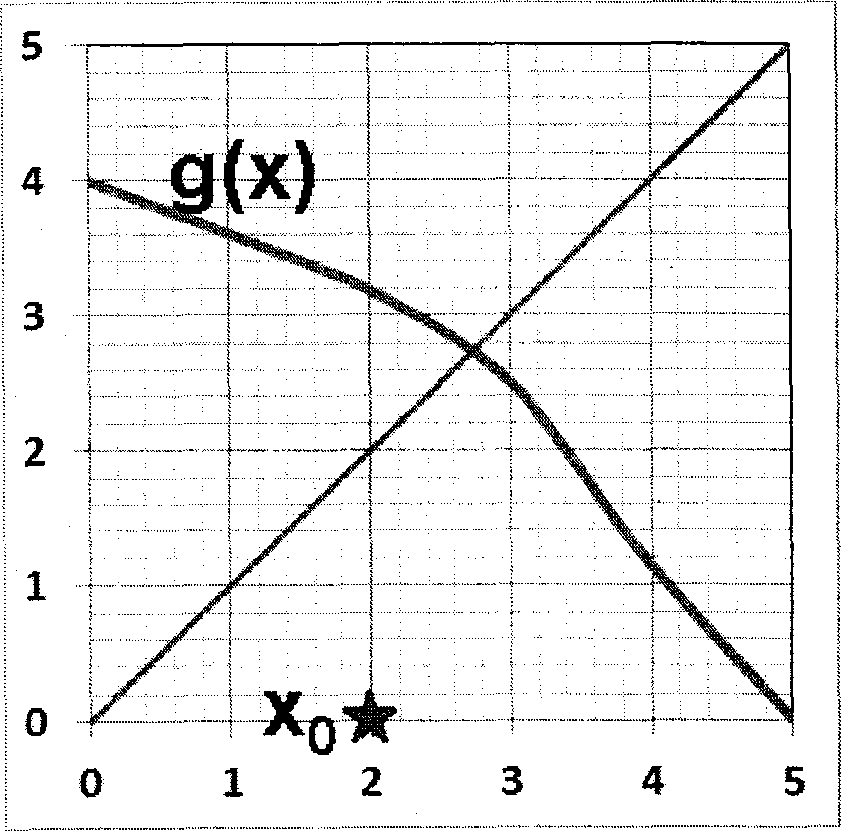
Q.31 Ammonia is synthesised at 200 bar and 773 K by the reaction N2 + 3H2 2NH3. The yield of ammonia is 0.45 mol/mol of fresh feed. Flow sheet for the process (along with available compositions) is shown below.
| |||||||||||||||
|
75 mol % H2 15 mol % N2 10 mol % inerts | |||||||||||||||
The single pass conversion for H2 in the reactor is 20 %. The amount of H2 lost in the purge as a PERCENTAGE of H2 in fresh feed is
(A) 10 (B) 20 (C) 45 (D) 55
Q.32 The following combustion reactions occur when methane is burnt.
CH4 + 202 - C02 + 2H20 2CH4 + 302 - 2CO + 4H20
20 % excess air is supplied to the combustor. The conversion of methane is 80 % and the molar ratio of CO to C02 in the flue gas is 1:3. Assume air to have 80 mol % N2 and rest 02. The 02 consumed as a PERCENTAGE of 02 entering the combustor is
(A) 20 (B) 62.5 (C) 80 (D) 83.3
Q.33 Consider a binary mixture of methyl ethyl ketone (component 1) and toluene (component 2). At 323 K the activity coefficients and are given by
In yj = x% (W\ -V2+ 42*1), In y2 = *? (Vi + 2 ~ 4W2 x2)
where x1 and x2 are the mole fractions in the liquid mixture, and y/x and \jf2 are parameters independent of composition. At the same temperature, the infinite dilution activity coefficients, y
and Y2 are given by In = 0.4 and In = 0.2. The vapour pressures of methyl ethyl ketone and toluene at 323 K are 36.9 and 12.3 kPa respectively. Assuming that the vapour phase is ideal, the equilibrium pressure (in kPa) of a liquid mixture containing 90 mol % toluene is (A) 19 (B) 18 (C) 16 (D) 15
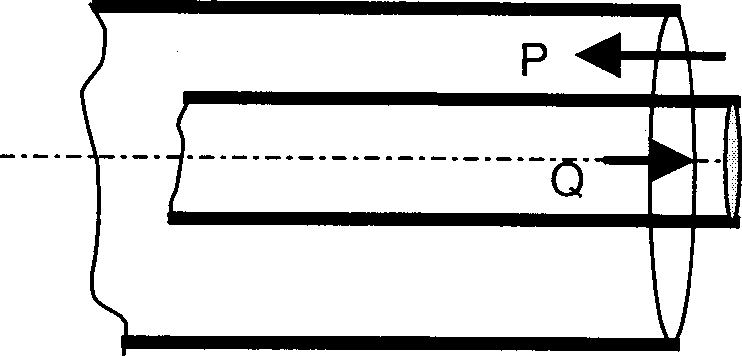
Densities of P and Q are 1000 and 800 kg/m3 respectively. The average velocities of the liquids P and Q are 1 and 2.5 m/s respectively. The inner diameters of the pipes are 0.31 and 0.1 m. Both pipes are 5 mm thick. The ratio of the Reynolds numbers ReP to ReQ is
Q.34 Two liquids (P and Q) having same viscosity are flowing through a double pipe heat exchanger as shown in the schematic below.
(A) 2.5 (B) 1.55 (C) 1 (D) 4
Q.35 The particle size distributions of the feed and collected solids (sampled for same duration) for a gas cyclone are given below.
|
Size range (|im) |
1-5 |
5-10 |
10-15 |
15-20 |
20-25 |
25-30 |
|
Weight of feed in the size range (g) |
2.0 |
3.0 |
5.0 |
6.0 |
3.0 |
1.0 |
|
Weight of collected solids in the size range (g) |
0.1 |
0.7 |
3.6 |
5.5 |
2.9 |
1.0 |
What is the collection efficiency (in PERCENTAGE) of the gas cyclone?
(A) 31 (B) 60 (C) 65 (D) 69
Q.36 A liquid is flowing through the following piping network. The length of pipe sections P, Q, R and S shown in the schematic are equal. The diameters of the sections P and R are equal and the diameter of the section Q is twice that of S. The flow is steady and laminar. Neglecting curvature and entrance effects, the ratio of the volumetric flow rate in the pipe section Q to that in S is
(A) 16 (B) 8 (C) 2 (D) 1
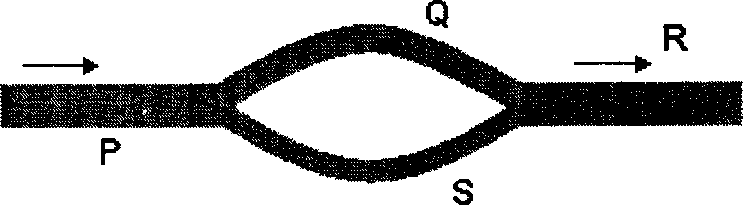
Oil at 120 C is used to heat water at 30 C in a 1-1 co-current shell and tube heat exchanger. The available heat exchange area is Si. The exit temperatures of the oil and the water streams are 90 C and 60 C respectively. The co-current heat exchanger is replaced by a 1-1 counter-current heat exchanger having heat exchange area S2. If the exit temperatures and the overall heat transfer coefficients are same, the ratio of Si to S2 is
(A) oo (B) 1.1 (C)0.91 (D) 0
Q.37
An aqueous sodium chloride solution (10 wt %) is fed into a single effect evaporator at a rate of 10000 kg/hr. It is concentrated to a 20 wt % sodium chloride solution. The rate of consumption of steam in the evaporator is 8000 kg/hr. The evaporator capacity (kg/hr) and economy are
Q.38
Q.39
(A) 5000, 0.625 (B) 10000, 0.625 (C) 5000, 1.6 (D) 10000, 1.6
Heat is generated uniformly within a solid slab. The slab separates fluid 1 from fluid 2. The heat transfer coefficients between the solid slab and the fluids are hi and h2 (h2 > h,) respectively. The steady state temperature profile (T vs. x) for one-dimensional heat transfer is CORRECTLY shown by
(A) (B)
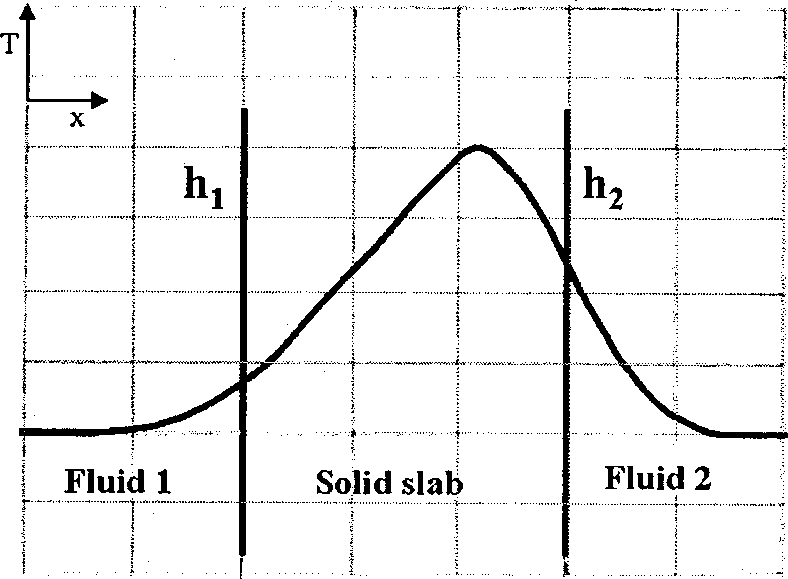
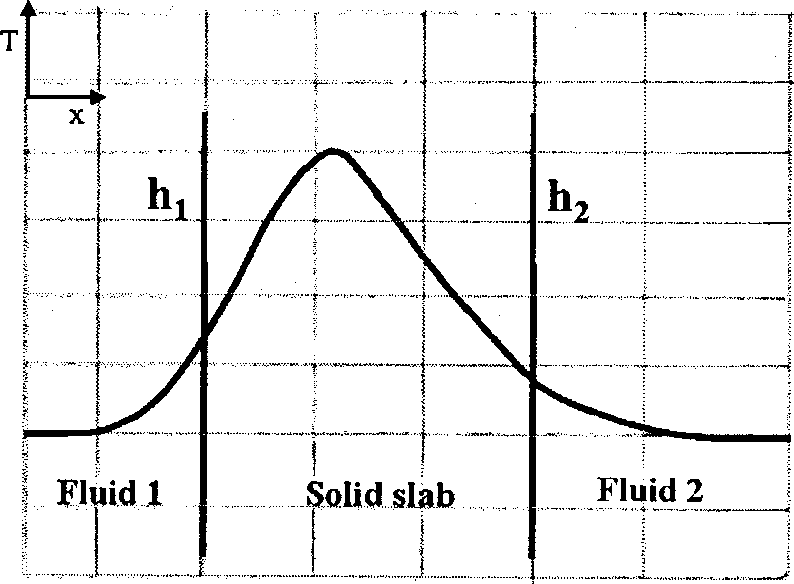
(C) (D)
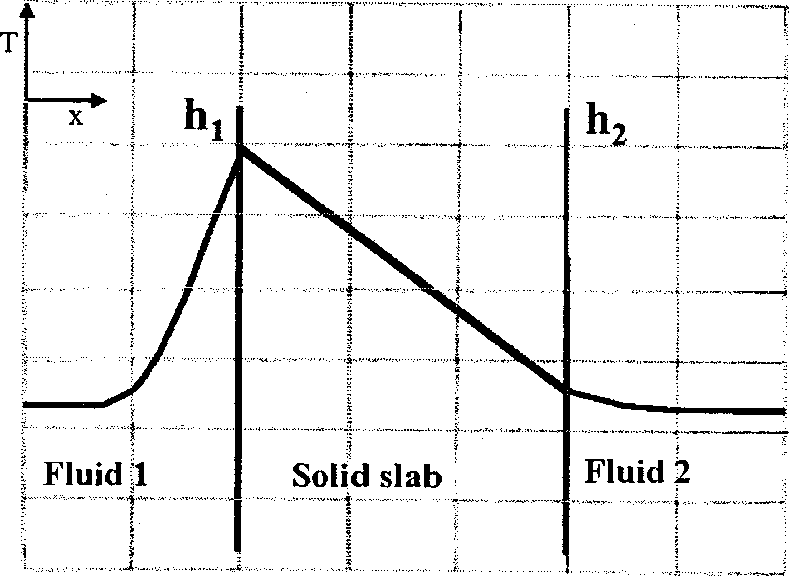
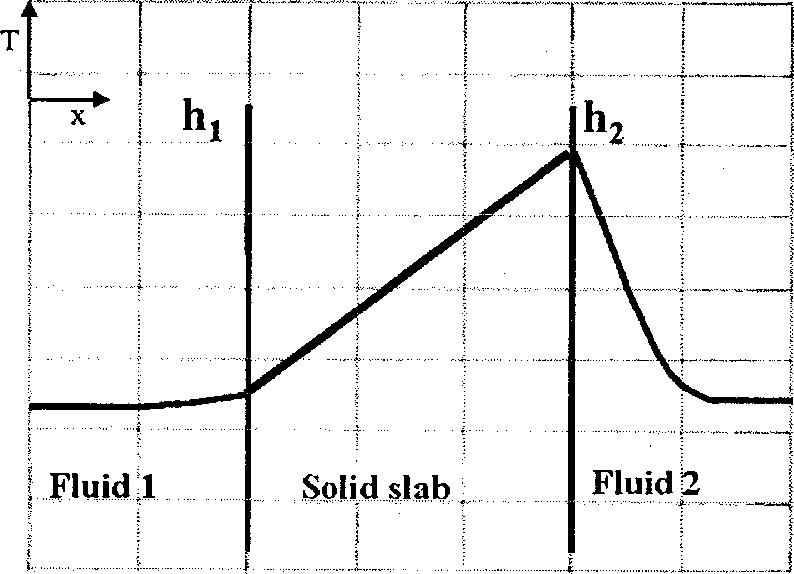
Q.40 A gas mixture is in contact with a liquid. Component P in the gas mixture is highly soluble in the liquid. Possible concentration profiles during absorption of P are shown in the choices, where
x : mole fraction of P in bulk liquid
y : mole fraction of P in bulk gas
x, : mole fraction of P at the interface in liquid
y, : mole fraction of P at the interface in gas
V *: equilibrium gas phase mole fraction corresponding to x,
The CORRECT profile is
|
(A) (B) |
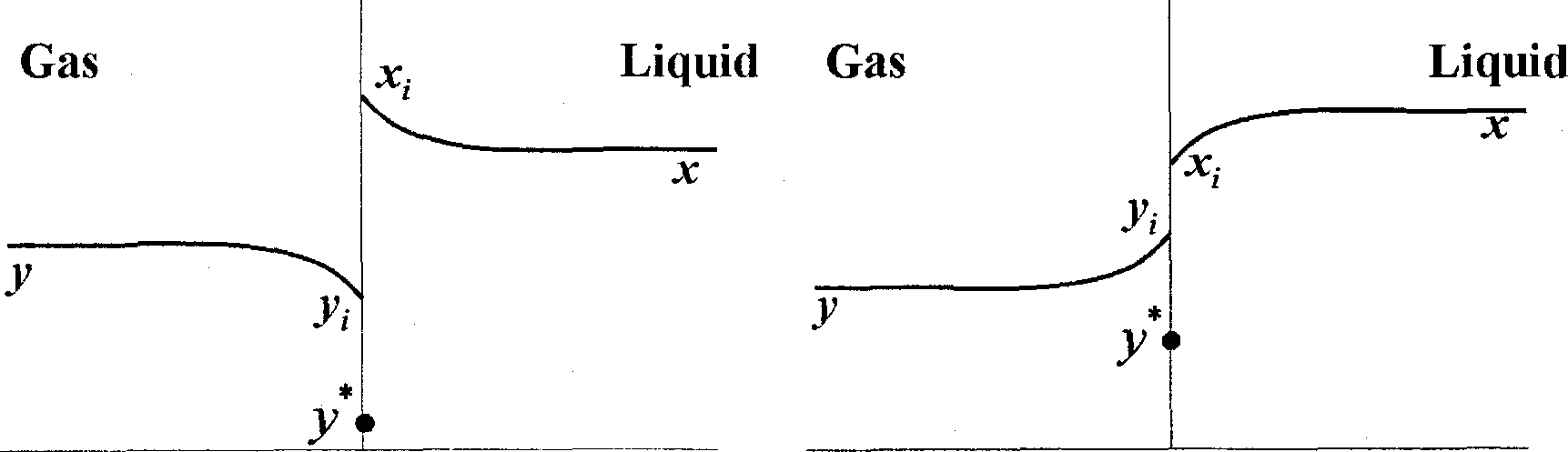 |
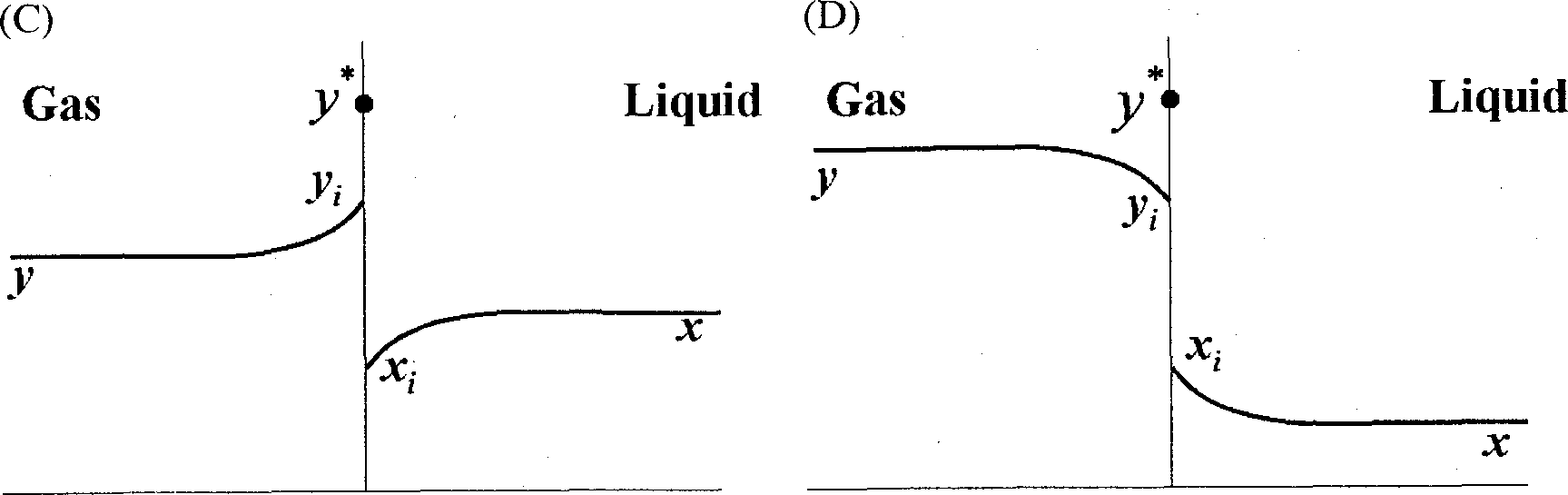
Q.41 A batch of 120 kg wet solid has initial moisture content of 0.2 kg water/kg dry solid. The exposed area for drying is 0.05 m2/kg dry solid. The rate of drying follows the curve given below.
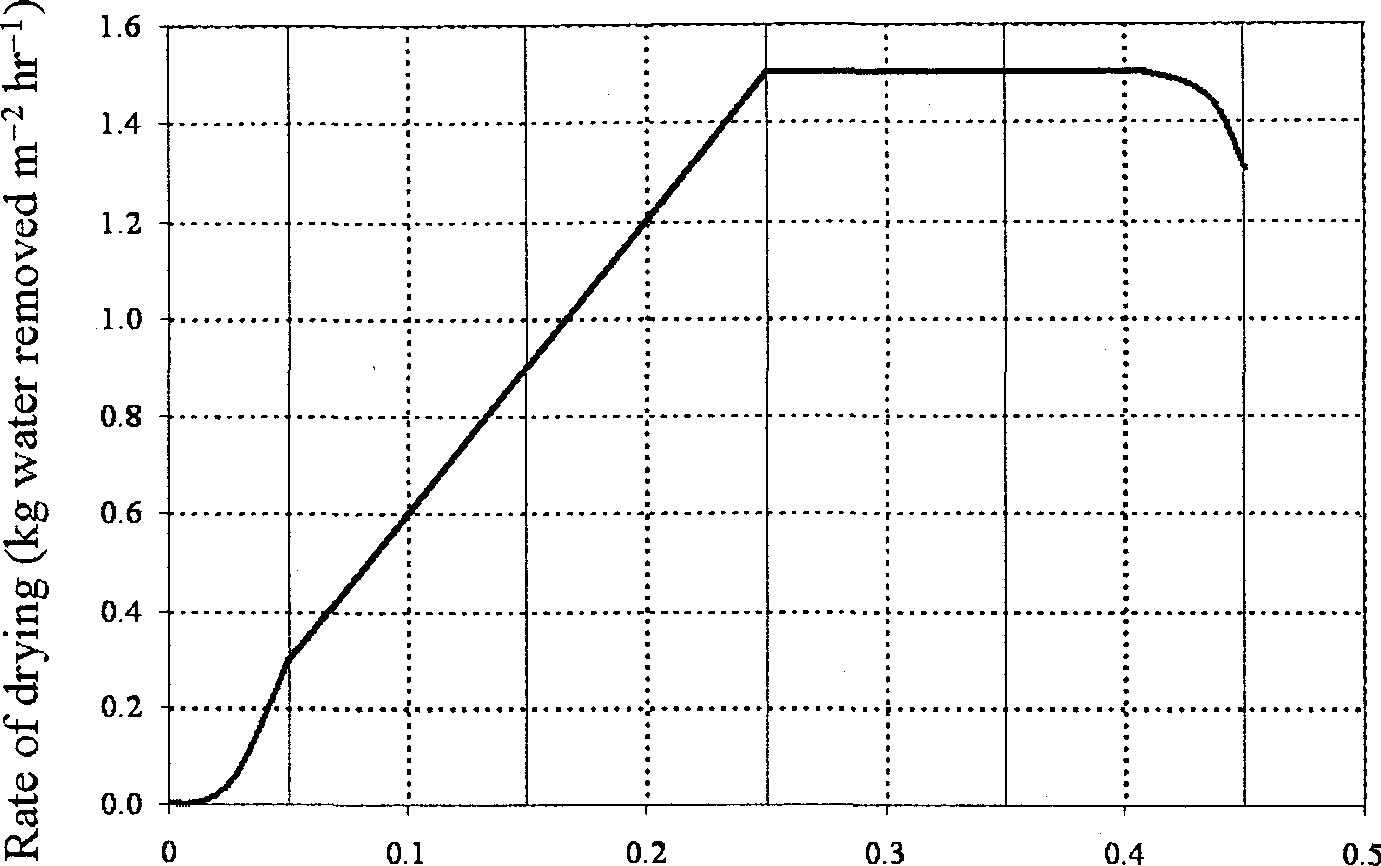 |
|
Moisture content, X (kg water/kg dry solid) |
The time required (in hours) for drying this batch to a moisture content of 0.1 kg water/kg dry solid is
(A) 0.033 (B) 0.43 (C) 0.6 (D)2.31
Q.42 For a first order catalytic reaction the Thiele modulus ((f>) of a spherical pellet is defined as
where
pp pellet density Rs = pellet radius
De = effective diffusivity k = first order reaction rate constant
If (f>> 5, then the apparent activation energy (Ea) is related to the intrinsic (or true) activation energy (E) as
(A) Ea = E5 (B) Ea = 0.5E (C) Ea = 2E (D) Ea = E2
Q.43 The following figures show the outlet tracer concentration profiles (c vs. t) for a pulse input.
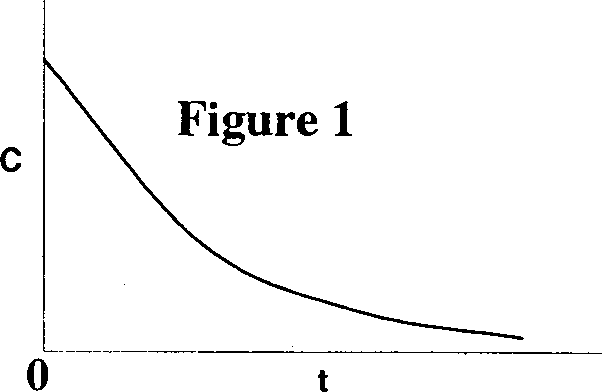
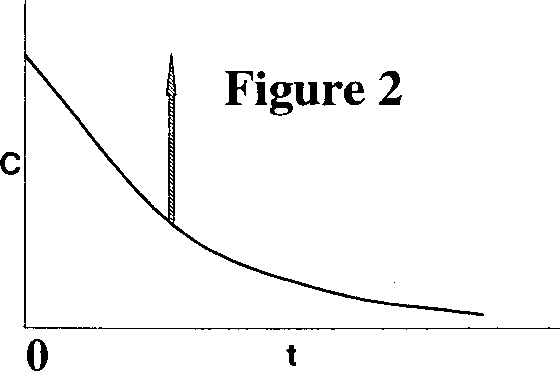
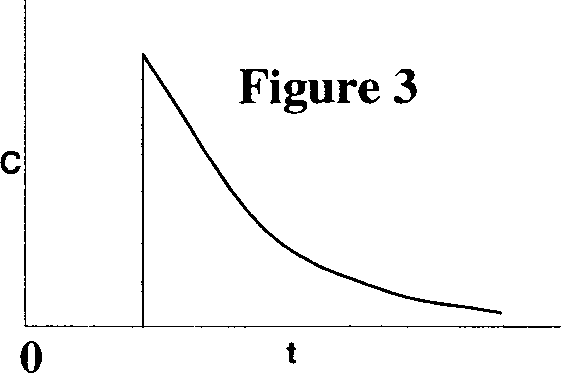
Match the figures in Group I with the reactor configurations in Group II.
P. Figure 1 I. PFR
Q. Figure 2 II. CSTR
R. Figure 3 III. PFR and CSTR in series
IV. PFR and CSTR in parallel
(A) P - II, Q - IV, R - III (B) P - IV, Q - III, R -1
(C)P-III, Q-IV, R-II (D)P-I, Q - III, R - II
Q.44 The following diagram shows a CSTR with two control loops. A liquid phase, endothermic reaction is taking place in the CSTR, and the system is initially at steady state. Assume that the changes in physical properties of the system are negligible.
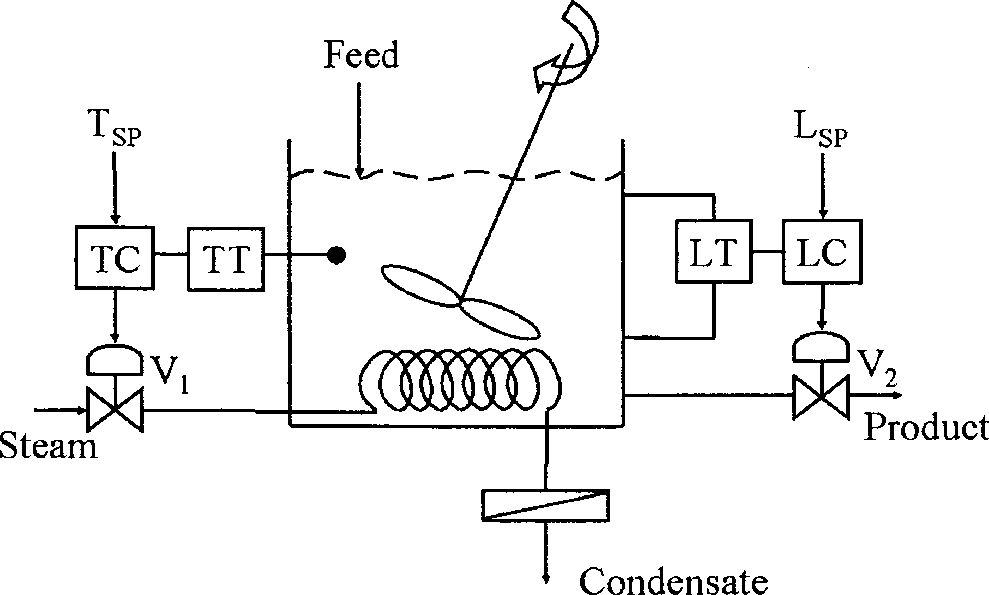
TC: Temperature controller, LC: Level controller, TT: Temperature transmitter, LT: Level transmitter, V) and V2: Control valves
Which ONE of the following statements is TRUE?
(A) Changing the level controller set point affects the opening of V2 ONLY
(B) Changing the temperature controller set point affects the opening of V2 ONLY
(C) Changing the temperature controller set point affects the opening of BOTH V, and V2
(D) Changing the level controller set point affects the opening of BOTH V[ and V2
Q.45 A process plant has a life of 7 years and its salvage value is 30 %. For what MINIMUM fixed-percentage factor will the depreciation amount for the second year, calculated by declining balance method be EQUAL to that calculated by the straight line depreciation method?
(A) 0.1 (B) 0.113 (C) 0.527 (D) 0.887
Q.46 A continuous fractionator system is being designed. The following cost figures are estimated for a reflux ratio of 1.4.
|
Fixed cost including all accessories (Rs.) for |
Operating cost (Rs./year) for | |||
|
column |
condenser |
reboiler |
condenser cooling water |
reboiler heating steam |
|
6x 106 |
2 x 106 |
4 x 106 |
8 x 106 |
1 x 106 |
The annualised fixed charge is 15 % of the fixed cost. The total annualised cost (in Rs.) is (A) 10.8 x 106 (B) 13.35 xlO6 (C) 15.9 x 106 (D)3.15xl06
|
P. Ammoxidation Q. Nitration R. Dehydrogenation Q.47 Match the reactions in Group I with the products in Group II. GROUP I S. Oxidation (A) P - III, Q - I, R - IV, S - II (C) P -1, Q - III, R - IV, S - II |
I. Aniline from benzene II. Benzoic acid from toluene III. Acrylonitrile from propylene IV. Styrene from ethylbenzene (B) P - IV, Q - I, R - III, S - II Common Data Questions Common Data for Questions 48 and 49: For a liquid flowing through a packed bed, the pressure drop per unit length of the bed is L AP _ 150/Vq (i-g)2 \.15pfV$(\-e) L (fisdp) e fisdpC where Vq is the superficial liquid velocity, is the bed porosity, dp is average particle size, </>s is particle sphericity, p f is liquid density and //y is liquid viscosity. Given data : dp = lxl0_3m, <ps = 0.8 , pj = 1000kg/ m3 , Mf = lxlO-3 kgm-1 s_1 , 3 2 particle density, pp= 2500kg/m and acceleration due to gravity, g =9.8m/s Q.48 When Vq is 0.005 m/s and = 0.5 , which ONE of the following is the CORRECT value for the ratio of the viscous loss to the kinetic energy loss? (A) 0.09 (B) 1.07 (C) 10.71 (D) 93 Q.49 On further increasing Vq , incipient fluidisation is achieved. Assuming that the porosity of the bed remains unaltered, the pressure drop per unit length (in Pa/m) under incipient fluidisation condition is (A) 3675 (B) 7350 (C) 14700 (D) 73501 Common Data for Questions 50 and 51: A binary feed mixture containing equimolar quantities of components S and T is to be distilled in a fractionating tower at atmospheric pressure. The distillate contains 96 mol % S. The g-line (feed line) intersects the equilibrium line at x = 0.46 and y = 0.66, where x and y' are mole fractions. Assume that the McCabe-Thiele method is applicable and the relative volatility is constant. Q.50 The MINIMUM reflux ratio is (A) 1.6 (B) 1.5 (C) 0.66 (D) 0.6 Q.51 The feed is (A) at dew point (B) at bubble point (C) superheated vapour (D) partially vapour Linked Answer Questions Linked Answer Questions 52 and 53: CH Q.52 In an aqueous solution, reaction P > Q occurs under isothermal conditions following first order kinetics. The feed rate is 500 cm3/min and concentration of P in the feed is 1.5 xlO""4 mol/cm3. The reaction is carried out in a 5 litre CSTR. At steady state, 60 % conversion is observed. The rate constant (in min-1) is (A) 0.06 (B) 0.15 (C) 0.21 (D) 0.28 Q.53 The 5 litre CSTR is replaced by five CSTRs in series. If the capacity of each new CSTR is 1 litre, then the overall conversion (in %) is (A) 65 (B) 67 (C) 73 (D) 81 Statement for Linked Answer Questions 54 and 55: A PID controller output p(t), in time domain, is given by t dg (f\ p(t)= 30 + 5e(t) + 1.25\e{t)dt + 15 0 dt where e(t) is the error at time t. The transfer function of the process to be controlled is t \ 10 p \ J ~ 200-jj ' measurement f the controlled variable is instantaneous and accurate. Q.54 The transfer function of the controller is 5(l22+45 + l) 5(l2s2 + 3s + l) (A) _V. ...............-1 (B) -L 3 s 3 5(l2s2+4s + l) 5(l252+3 + l) (O -L (D) -L 45 As Q.55 The characteristic equation of the closed loop is (A) 6s2 + 1025 + 1-0 (C) 10052-1965-25 = 0 (B) 70052 +1025+ 25 = 0 General Aptitude (GA) Questions Q. 56 - Q. 60 carry one mark each. Q.56 Choose the most appropriate word(s) from the options given below to complete the following sentence. I contemplated_Singapore for my vacation but decided against it. (A) to visit (B) having to visit (C) visiting (D) for a visit Q.57 If Log (P) = (l/2)Log (Q) = (1/3) Log (R), then which of the following options is TRUE? (A)P2=Q3R2 (B)Q2=PR (C) Q2=R3P (D) r = p2q2 Q.58 Which of the following options is the closest in the meaning to the word below: Inexplicable (A) Incomprehensible (B) Indelible (C) Inextricable (D) Infallible Q.59 Choose the word from the options given below that is most nearly opposite in meaning to the given word: Amalgamate (A) merge (B) split (C) collect (D) separate Q.60 Choose the most appropriate word from the options given below to complete the following sentence. If you are trying to make a strong impression on your audience, you cannot do so by being understated, tentative or_. (A) hyperbolic (B) restrained (C) argumentative (D) indifferent Q. 61 to Q. 65 carry two marks each. Q.61 The variable cost (V) of manufacturing a product varies according to the equation V= 4q, where q is the quantity produced. The fixed cost (F) of production of same product reduces with q according to the equation F = 100/q. How many units should be produced to minimize the total cost (V+F)? (A) 5 (B) 4 (C) 7 (D) 6 Q.62 P, Q, R and S are four types of dangerous microbes recently found in a human habitat. The area of each circle with its diameter printed in brackets represents the growth of a single microbe surviving human immunity system within 24 hours of entering the body. The danger to human beings varies proportionately with the toxicity, potency and growth attributed to a microbe shown in the figure below: 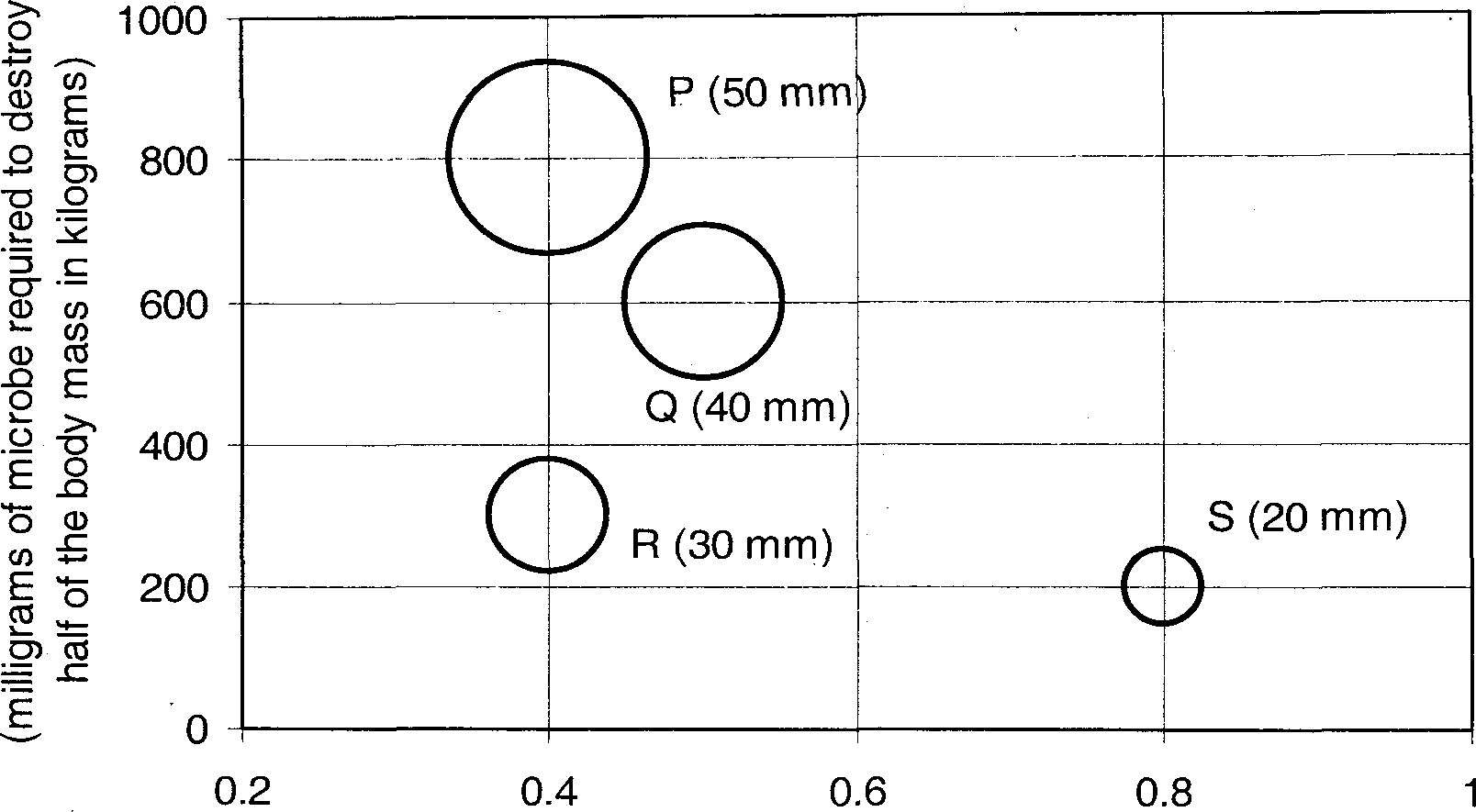
Potency (Probability that microbe will overcome human immunity system) A pharmaceutical company is contemplating the development of a vaccine against the most dangerous microbe. Which microbe should the company target in its first attempt? (A) P (B) Q (C) R (D) S Q.63 Few school curricula include a unit on how to deal with bereavement and grief, and yet all students at some point in their lives suffer from losses through death and parting. Based on the above passage which topic would not be included in a unit on bereavement? (A) how to write a letter of condolence (B) what emotional stages are passed through in the healing process (C) what the leading causes of death are (D) how to give support to a grieving friend Q.64 A container originally contains 10 litres of pure spirit. From this container 1 litre of spirit is replaced with 1 litre of water. Subsequently, 1 litre of the mixture is again replaced with 1 litre of water and this process is repeated one more time. How much spirit is now left in the container? (A) 7.58 litres (B) 7.84 litres (C) 7 litres (D) 7.29 litres Q.65 A transporter receives the same number of orders each day. Currently, he has some pending orders (backlog) to be shipped. If he uses 7 trucks, then at the end of the 4th day he can clear all the orders. Alternatively, if he uses only 3 trucks, then all the orders are cleared at the end of the 10th day. What is the minimum number of trucks required so that there will be no pending order at the end of the 5th day? (A) 4 (B) 5 (C) 6 (D) 7 END OF THE QUESTION PAPER CH 20/24
|
