Indian Institute of Technology Madras (IIT-M) 2012 GATE Chemistry with Answer Key - Question Paper
2012 CHEMISTRY - CY
Duration: Three Hours Maximum Marks: 100
Read the following instructions carefully.
1. Do not open the seal of the Question Booklet until you are asked to do so by the invigilator.
2. Take out the Optical Response Sheet (ORS) from this Question Booklet without breaking the seal and read the instructions printed on the ORS carefully.
3. On the right half of the ORS, using ONLY a black ink ball point pen, (i) darken the bubble corresponding to your test paper code and the appropriate bubble under each digit of your registration number and (ii) write your registration number, your name and name of the examination centre and put your signature at the specified location.
4. This Question Booklet contains 20 pages including blank pages for rough work. After you are permitted to open the seal, please check all pages and report discrepancies, if any, to the invigilator.
5. There are a total of 65 questions carrying 100 marks. All these questions are of objective type. Each question has only one correct answer. Questions must be answered on the left hand side of the ORS by darkening the appropriate bubble (marked A, B, C, D) using ONLY a black ink ball point pen against the question number. For each question darken the bubble of the correct answer. More than one answer bubbled against a question will be treated as an incorrect response.
6. Since bubbles darkened by the black ink ball point pen cannot be erased, candidates should darken the bubbles in the ORS very carefully.
7. Questions Q.1 - Q.25 carry 1 mark each. Questions Q.26 - Q.55 carry 2 marks each. The 2 marks questions include two pairs of common data questions and two pairs of linked answer questions. The answer to the second question of the linked answer questions depends on the answer to the first question of the pair. If the first question in the linked pair is wrongly answered or is unattempted, then the answer to the second question in the pair will not be evaluated.
8. Questions Q.56 - Q.65 belong to General Aptitude (GA) section and carry a total of 15 marks. Questions Q.56 - Q.60 carry 1 mark each, and questions Q.61 - Q.65 carry 2 marks each.
9. Unattempted questions will result in zero mark and wrong answers will result in NEGATIVE marks. For all 1 mark questions, % mark will be deducted for each wrong answer. For all 2 marks questions, 2A mark will be deducted for each wrong answer. However, in the case of the linked answer question pair, there will be negative marks only for wrong answer to the first question and no negative marks for wrong answer to the second question.
10. Calculator is allowed whereas charts, graph sheets or tables are NOT allowed in the examination hall.
11. Rough work can be done on the question paper itself. Blank pages are provided at the end of the question paper for rough work.
12. Before the start of the examination, write your name and registration number in the space provided below using a black ink ball point pen.
|
Name | ||||||||
|
Registration Number |
CY | |||||||
RT 1. At T = 298 K, -2.303 = 0.0591
Some Useful Data
F
2. Atomic Numbers
H 1
B 5
C 6
N 7
O 8
P 15
Ca 20
Cr 24
Mn 25
Fe 26
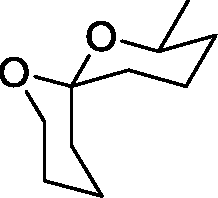
Co 27
Ni 28
Zn 30
Ru 44
3. Atomic weights (to the nearest integer)
H 1 C 12 N 14
O 16 Ca 40
Q. 1 - Q. 25 carry one mark each
Q.1 In the proton decoupled 13C NMR spectrum of 7-norbomanone, the number of signals obtained is
(A) 7 (B) 3 (C) 4
(D) 5
Q.2 Identify the most probable product in the given reaction
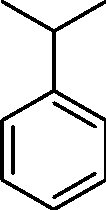 |
02/ benzoyl peroxide |
Product
(A)
(B)
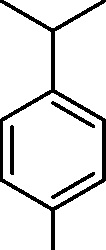 |
|
OOH |
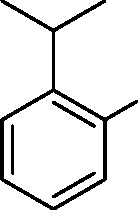 |
OOH |
(D)
(C)
OOH
 |
OOH |
Q.3 In the cyclization reaction given below, the most probable product formed is
Br
NaH Product
VX/OH
OH
(B)
(C) (D)
Q.4 If Ay and Apy are the uncertainties in the y-coordinate and the y component of the momentum of a
h
particle respectively, then, according to uncertainty principle AvApv is (ti = - and h is Plancks constant)
2 n (D) > ti/2
(B) >h/2
The average length of a typical a-helix comprised of 10 amino acids is
Q.5
(A) 10 A (B) 15 A (C) 36 A (D) 54 A
Q.6 Number of thymine residues in a 5000 kb DNA containing 23% guanine residues is
(A) 2.70 x 106 (B) 2.70 x 107 (C) 1.35 x 106 (D) 1.35 x 107
_CHEMISTRY - CY
Shown below is a Hammett plot obtained for the reaction
0 o
1 h2o A
ArCI ---- ArOH
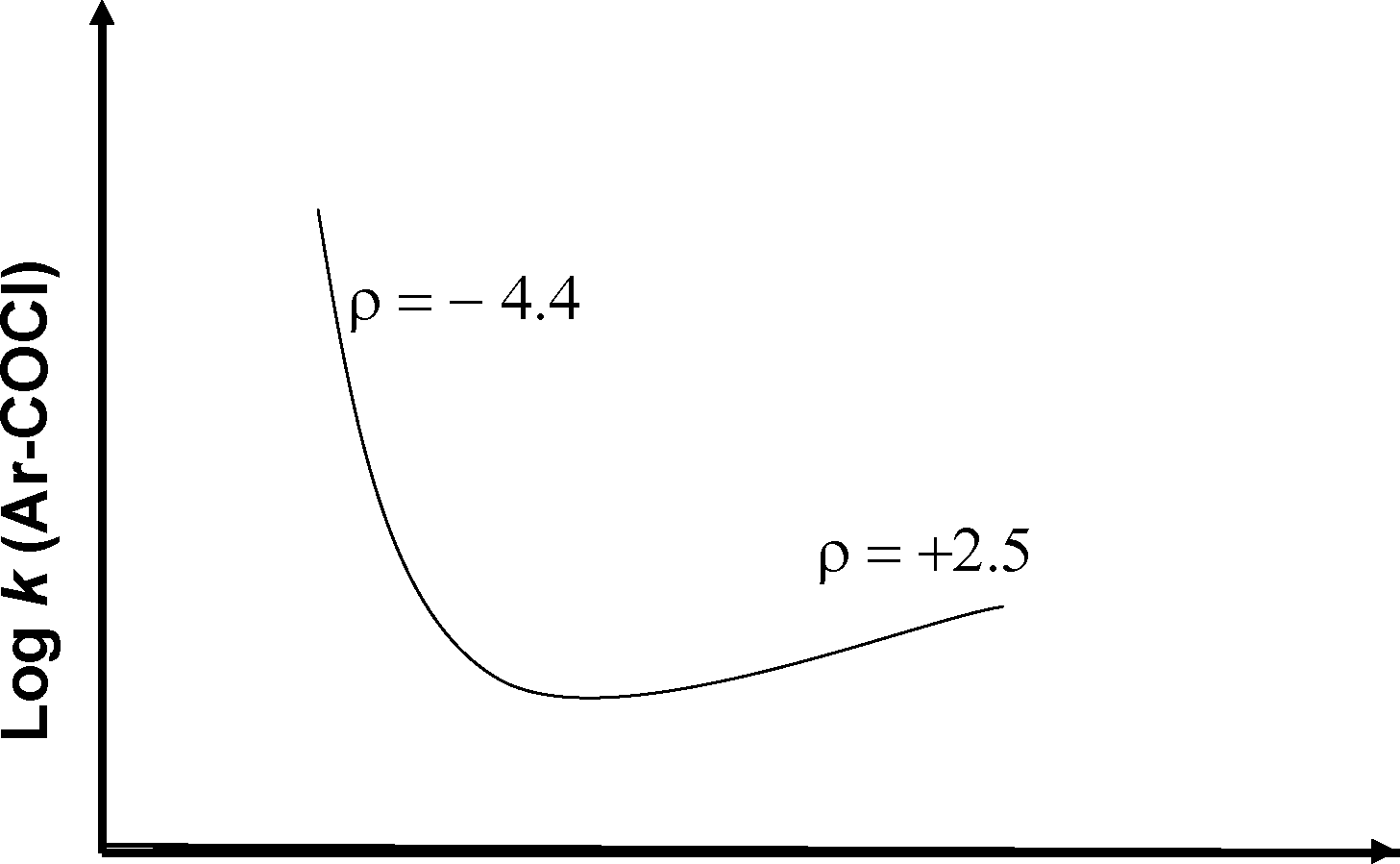
a
The change in slope of the plot indicates that
(A) the reaction does not follow linear free energy relationship
(B) electrons are being withdrawn from the transition state in the mechanism
(C) electrons are being donated to the transition state in the mechanism
(D) the mechanism of the reaction is changing
The ratio of relative intensities of the two molecular ion peaks of methyl bromide (CH3Br) in the mass spectrum is
Q.8
(A) M+ : (M+2)+ = 1:3
(B) M+ : (M+2)+ = 3:1
(C) M+ : (M+2)+ = 1:1
(D) M+ : (M+2)+ = 1:2
Q.9 A disaccharide that will not give Benedicts test and will not form osazone is
(A) maltose (B) lactose (C) cellobiose (D) sucrose
Q.10 Choose the allowed transition
1v + . 3
(A) %+ %+ (B) %+ % (C) 1Sg+ V (D) 1Sg+ 1Su
Q.11 The angular part of the wavefunction for the electron in a hydrogen atom is proportional to sin2 0 cosO e2. The values of the azimuthal quantum number (l) and the magnetic quantum number (m) are, respectively
(A) 2 and 2
(B) 2 and -2
(C) 3 and 2
(D) 3 and -2
Q12 Let and denote the wavefunctions of the 2px and 2pz orbitals of carbon, respectively, and and represent the wavefunctions of the 2px and 2pz orbitals of oxygen, respectively. If C and c2 are constants used in linear combinations and the CO molecule is oriented along the
z axis, then, according to molecular orbital theory, the n-bonding molecular orbital has a wavefunction given by
(B) c + c2
(D) C1 + C2
Q.13 The bond that gives the most intense band in the infrared spectrum for its stretching vibration is
(A) C-H (B) N-H (C) O-H (D) S-H
Q.14 If xA and xB are the respective mole fractions of A and B in an ideal solution of the two and TA, TB, T are the fusion temperatures of pure A, pure B and the ideal solution respectively, then
A
-AH
1 1
fus( B)
(A) 1 - xB = exp
(B) 1 - xB = exp
(C) 1 - xB = exp
(D) 1 - xB = exp
R
T T
V1 tb
AH
fus( A)
R
T T
V T ta y
1_J_
T T
VT tb y
AH
fus( B)
R
-AH
1-1
T T
v T ta y
fus( A)
R
Q.15 For a reaction involving two steps given below
G
G + H
2H
P
First step: Second step:
assume that the first step attains equilibrium rapidly. The rate of formation of P is proportional to
1/2
3/2
(A) [G]
(B) [G]
(D) [G]
(C) [G]2
Q.16 A metal chelate that can be used for separation and quantitative analysis of aluminium ions by gas chromatography is
(A) EDTA
(C) dinonyl phthalate
(B) ethylene glycol (D) trifluoroacetylacetone
Q.17 The enthalpies of hydration of Ca2+, Mn2+ and Zn2+ follow the order
,2+
Q.18 The number of terminal carbonyl groups present in Fe2(CO)9 is
(D) 3
(A) 2 (B) 5 (C) 6
Q.19 Among the following substituted silanes, the one that gives cross-linked silicone polymer upon hydrolysis is
(A) (CHs)4Si
(B) CHsSiCls
(C) (CH3)2SiCl2
(D) (CH3)3SiCl
Q.20 The plot of xT versus T (where x is molar magnetic susceptibility and T is the temperature) for a paramagnetic complex which strictly follows Curie equation is
(A)
(B)
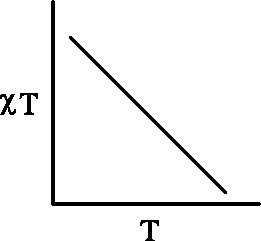
XT
(C)
(D)
XT
XT
Q.21 Among the following donors, the one that forms most stable adduct with the Lewis acid B(CH3)3 is
(A) 4-methylpyridine
(B) 2,6-dimethylpyridine
(C) 4-nitropyridine
(D) 2,6-di-tert-butylpyridine
Q.22 The complex with inverse-spinel structure is
(A) Co3O4 (B) Fe3O4 (C) MgAlO4 (D) Mn3O4
2012_CHEMISTRY - CY
Q.23 The IUPAC nomenclature of Na[PCl6] is
(A) sodium hexachlorophosphine(V)
(B) sodium hexachlorophosphate(V)
(C) sodium hexachlorophosphine
(D) sodium hexachlorophosphite(V)
Q.24 An intermediate formed during the hydroformylation of olefins using Co2(CO)8 as catalyst is (A) HCo(CO)6 (B) H4Co(CO)3 (C) H2Co(CO)4 (D) HCo(CO)4
Q.25 The order of polarity of NH3, NF3 and BF3 is
(A) NH3 < NF3 < BF3
(B) BF3 < NF3 < NH3
(C) BF3 < NH3 < NF3
(D) NF3 < BF3 < NH3
Q. 26 to Q. 55 carry two marks each.
Q.26 From a carboxymethyl-cellulose column at pH 6.0, arginine, valine and glutamic acid will elute in the order
(A) arginine, valine, glutamic acid
(B) arginine, glutamic acid, valine
(C) glutamic acid, arginine, valine
(D) glutamic acid, valine, arginine
Q.27 Symmetry operations of the four C2 axes perpendicular to the principal axis belong to the same class in the point group(s)
(A) D4
(B) D
(D) D4h and D
(C) D
Q.28 At 298 K, the EMF of the cell
Pt |H2(1 bar) |H+(solution)||CF |Hg2Cl2 |Hg
is 0.7530 V. The standard potential of the calomel electrode is 0.2802 V. If the liquid junction potential is zero, the pH of the solution is
(A) 4.7 (B) 7.4 (C) 8.0 (D) 12.7
_CHEMISTRY - CY
The wavefunction of a 1-D harmonic oscillator between x = +x> and x = w is given by
/(x) = N(2x1 1)e_x . The value of Nthat normalizes the function /(x) is (Given: Jx2V*A= 135'2~1))
W
(B)
(D)
(C)
Consider the reaction
Q.30
The molecular diameters of H2 and C2H4 are 1.8 A and 3.6 A respectively. The pre-exponential factor in the rate constant calculated using collision theory in m3( mole) 1 s_1 is approximately
1
2
8KT
Na = 1.11 x 10 m(mole) s , where the symbols have
(For this reaction at 300 K, their usual meanings)
(A) 2.5 x108
14
17
23
(B) 2.5 x10:
(C) 9.4 x10:
(D) 9.4 x10
The molecular partition function of a system is given by
Q.31
8w mkBT h
q(T) =
hc
, where the symbols have their usual meanings.
The heat capacity at constant volume for this system is
(A) 3R
(B) 6R
(C) 9R/2
(D) 3R/2
Consider the phase diagram given below.
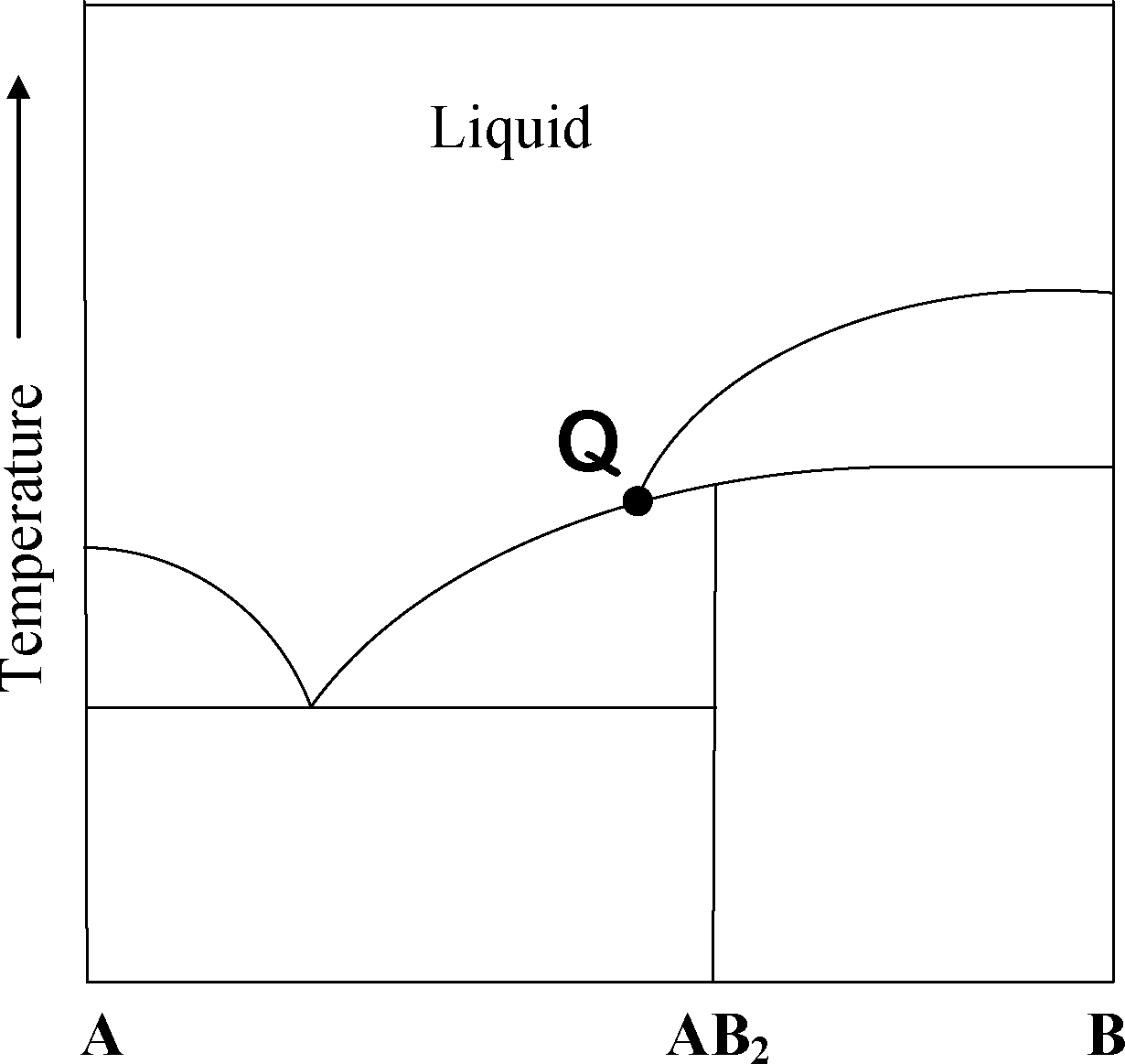
At the intersection point Q the phases that are in equilibrium are
(A) solid A, solid B and solid AB2
(B) solid A, solid AB2 and liquid
(C) solid B, solid AB2 and liquid
(D) solid A, solid B, solid AB2 and liquid
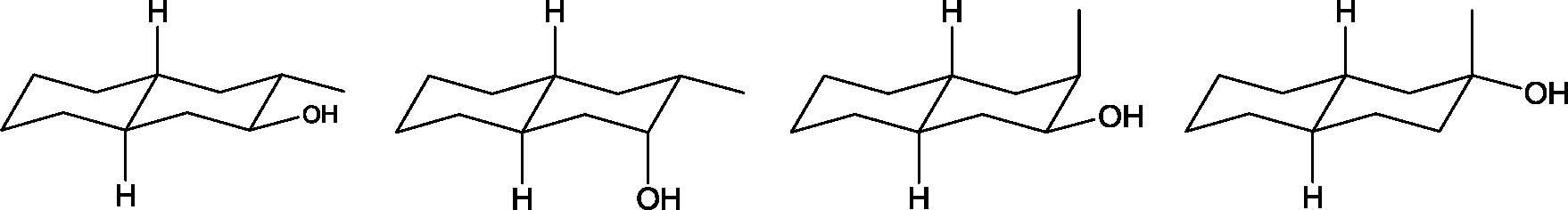
Identify the product from the following reaction
Q.33
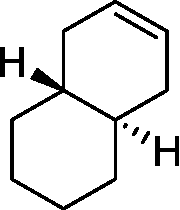
Product
|
(A) (9-BBN = 9-Borabicyclo[31]nonane) (B) (C) (D) |
 |
Q.34 The product from the following reaction is
hv
Product
(A)
(B)
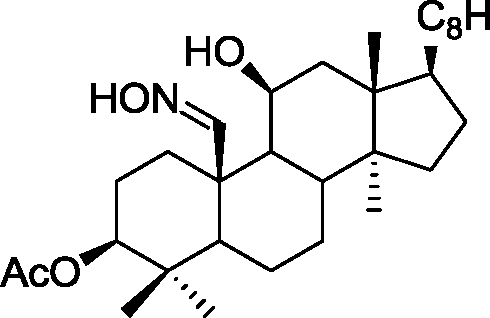
(C)
(D)
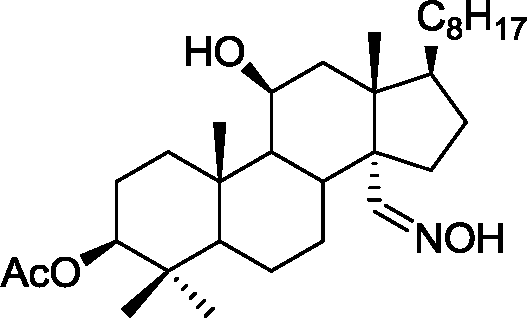
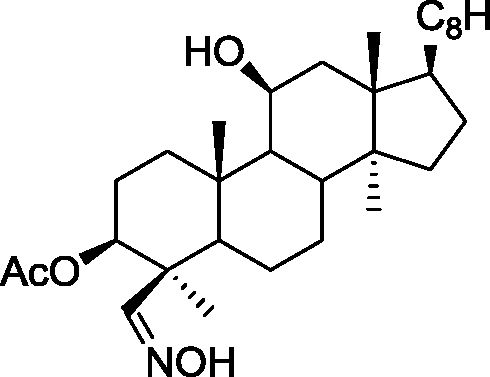
Q.35 The acid catalyzed cyclization of 5-ketodecan-1,9-diol is given below HQ. .___. ___ .___ ___ P-TSA, benzene, heat
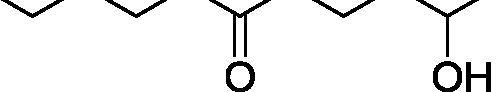
Spiroketal
(p-TSA = p-toluenesulfonic acid)
The most predominant spiroketal is
(A) (B)
(C)
(D)
Q.36 For a face centered cubic lattice, the Miller indices for the first Braggs peak (smallest Bragg angle) are
(A) 0 0 2
(B) 1 1 1
(C) 0 0 1
(D) 1 1 0
Q.37 For the titration of a 10 mL (aq) solution of CaCO3, 2 mL of 0.001 M Na2EDTA is required to reach the end point. The concentration of CaCO3 (assume molecular weight of CaCO3 = 100) is
(A) 5 x 10-4 g/mL (C) 5 x 10-5 g/mL
(B) 2 x 10-4 g/mL (D) 2 x 10-5 g/mL
KOH-EtOH
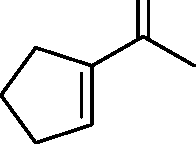
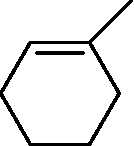
|
Q.38 In the reaction O | |
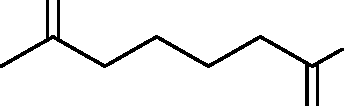 |
H |
Product
the product formed is
(C)
(D)
(A) (B)
|
O |
 |
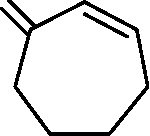
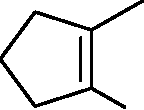
CHO
Q.39 In the reaction given below, identify the product
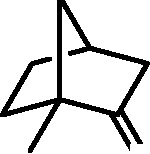
1. CH2=CHMgBr, THF
Product
2. H30+
O 3. excess CH3C(OMe)3, p-TSA, heat
(p-TSA = p-toluenesulfonic acid; THF = tetrahydrofuran)
(B)
OCMe(OMe)2
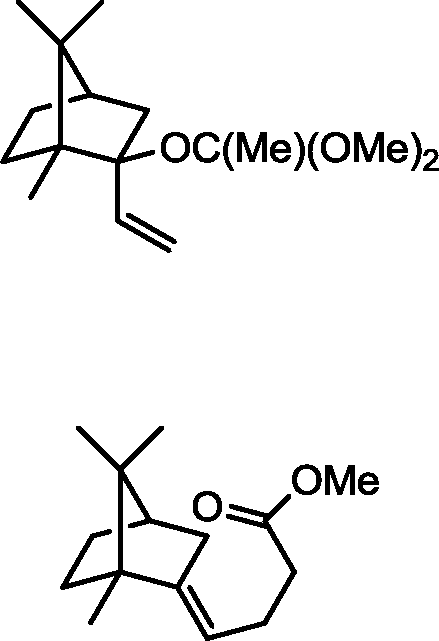
(A)
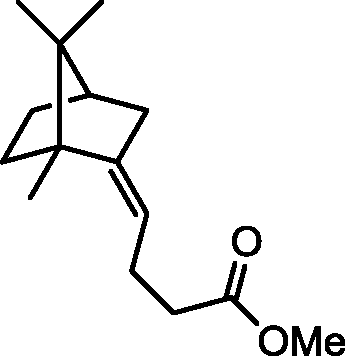
(C)
(D)
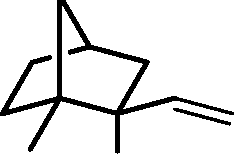
|
Q.40 Consider the following pairs of complexes [CoF(NH3)5]2+ and [Cr(OH2)6]2+ [Co(NH3)5 (OH2)]3+ and [Cr(OH2)6]2+ [Co(NH3)6]3+ and [Cr(OH2)6]2+ [CoI(NH3)5]2+ and [Cr(OH2)6]2+ The electron transfer rate will be fastest in the pair (A) [CoF(NH3)5]2+ and [Cr(OH2)6]2+ (C) [Co(NH3)6]3+ and [Cr(OH2)6]2+ |
(B) [Co(NH3)5 (OH2)]3+ and [Cr(OH2)6]2 (D) [CoI(NH3)5]2+ and [Cr(OH2)6]2+ |
Q.41 The extent of Mossbauer quadrupole splitting of iron follows the order
(A) FeCO > K2[Fe(CN)5(NO)] > FeCUO
(B) K2[Fe(CN)5(NO)] > FeCl24H2O > FeCO
(C) FeCl36H2O > K2[Fe(CN)5(NO)] > FeCl24H2O
(D) FeCl24H2O > FeCl36H2O > K2[Fe(CN)5(NO)]
Q.42 Hemoglobin is an oxygen carrying protein. The correct statement about oxy-hemoglobin is that
(A) the metal is low-spin in +3 oxidation state while dioxygen is in O2- form
(B) the metal is high-spin in +3 oxidation state while dioxygen is in O2- form
(C) the metal is low-spin in +3 oxidation state while dioxygen is in neutral form
(D) the metal is high-spin in +3 oxidation state while dioxygen is in neutral form
Q.43 If a mixture of NaCl, conc. H2SO4 and K2Cr2O7 is heated in a dry test tube, a red vapour (P) is formed. This vapour (P) dissolves in aqueous NaOH to form a yellow solution, which upon treatment with AgNO3 forms a red solid (Q). P and Q are, respectively
(A) CrO2Cl2 and Ag2O7
(B) Na2[CrOCl5] and Ag2CrO4
(C) Na2[CrOCl5] and Ag2Cr2O7
(D) CrO2Cl2 and Ag2CrO4
Q.44 For the following reaction
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 8H2O + 10CO2
E(MnO47Mn2+) = +1.51 V and E(CO2/H2C2O4) = - 0.49 V.
At 298 K, the equilibrium constant is
(A) 10500 (B) 10338 (C) 1038 (D) 10833
Q.45 The ground states of high-spin octahedral and tetrahedral Co(II) complexes are, respectively
(A) 4T2g and % (B) 4T and %
(C) 3Tjg and % (D) 4T and 3T
Q.46 The INCORRECT statement about Zeises salt is
(A) Zeises salt is diamagnetic
(B) The oxidation state of Pt in Zeises salt is +2
(C) All the PtCl bond lengths in Zeises salt are equal
(D) CC bond length of ethylene moiety in Zeises salt is longer than that of free ethylene molecule
Q.47 The number of possible isomers for the square planar mononuclear complex [(NH3)2M(CN)2] of a metal M is
(A) 2 (B) 4 (C) 6 (D) 3
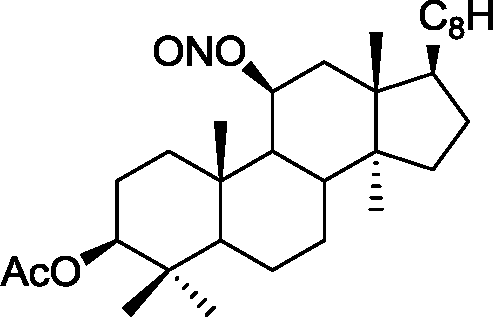
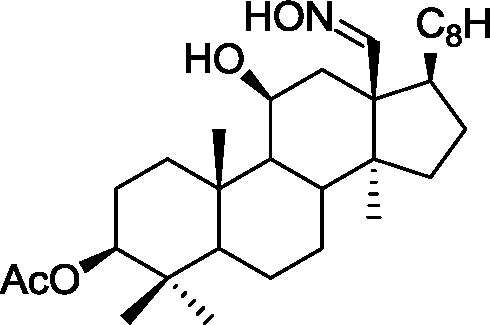
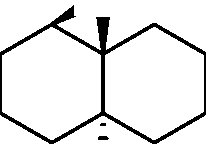
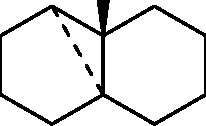
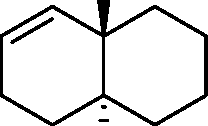
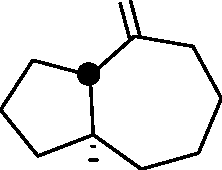
Common Data for Questions 48 and 49: Consider the reaction sequence shown below:
|
2. TsCI, Pyrdine | 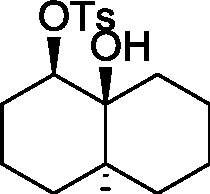 |
f-BuOK/f-BuOH |
|
H | ||
 |
|
H |
1.X
Product
TsCl = -toluenesulfonyl chloride
|
Q.48 The oxidant X used in step 1 is (A) CrO3 (C) NaIO4 |
(B) OsO4 (D) m-CPBA followed by NaOH |
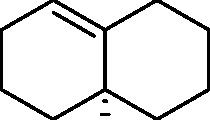
Q.49 The product is
(A)
(B)
(C)
(D)
|
0 |
 |
|
H |
|
OH |
 |
|
OH |
 |
|
H |
 |
|
H |
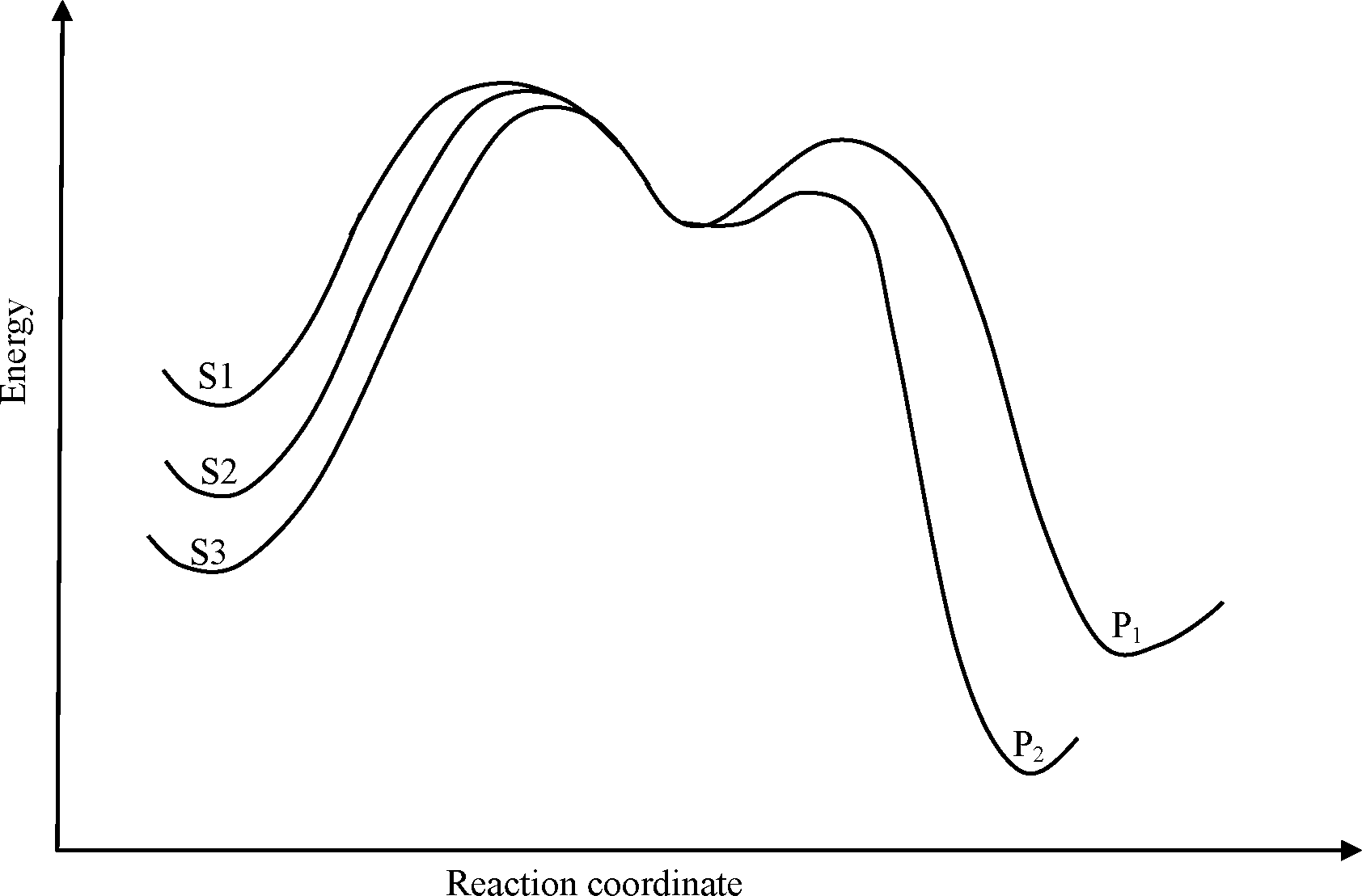
Common Data for Questions 50 and 51: Consider the E1 reaction of tert-amyl halides from the energy profile given below.
\
>
-X
E1 reaction
R-X
M
N
Q.50 In the above reaction, X = Cl, Br or I. Based on the graph, identify the alkyl halides (R-X) as S1, S2 and S3
|
(A) S1 = R-Cl, S2 = R-Br and S3 = R-I (C) S1 = R-Cl, S2 = R-I and S3 = R-Br |
(B) S1 = R-I, S2 = R-Br and S3 = R-Cl (D) S1 = R-I, S2 = R-Cl and S3 = R-Br |
Q.51 Identify product Pi and its yield relative to P2
|
(A) P1 is M and is the major product (C) P1 is N and is the major product |
(B) P1 is N and is the minor product (D) P1 is M and is the minor product |
Statement for Linked Answer Questions 52 and 53: A 20491 cm-1 laser line was used to excite oxygen molecules (made of 16O only) to obtain the rotational Raman spectrum. The resulting rotational Raman spectrum of oxygen molecule has the first Stokes line at 20479 cm-1.
Q.52 The rotational constant (usually denoted as B) for the oxygen molecule is
(A) 1.2 cm-1 (B) 2.0 cm-1 (C) 3.0 cm-1 (D) 6.0 cm-1
Q.53 The next rotational Stokes line is expected at
(A) 20467 cm-1 (B) 20469 cm-1 (C) 20471 cm-1 (D) 20475 cm-1
Statement for Linked Answer Questions 54 and 55: Huckel molecular orbital theory can be applied to the allene radical
CH2=CH-CH2
|
Q.54 The secular determinant (where a , fi and E have their usual meanings) is given by (A) (B) | |||||||||||||||||||||
|
|
(C) (D) | |||||||||||||||||||||
|
Q.55 The possible values of E are
(A) a + yjip, a, a-42p (B) a + 2yf2fi, a, a-22fi
(C) a + fi, a, a-fi (D) a + 2fi, a, a-2fi
General Aptitude (GA) Questions (Compulsory) Q. 56 - Q. 60 carry one mark each.
Q.56 If (1.001)1259 = 3.52 and (1.001)2062 = 7.85, then (1.001)3321 =
(A) 2.23 (B) 4.33 (C) 11.37 (D) 27.64
Q.57 One of the parts (A, B, C, D) in the sentence given below contains an ERROR. Which one of the following is INCORRECT?
I requested that he should be given the driving test today instead of tomorrow.
(A) requested that
(B) should be given
(C) the driving test
(D) instead of tomorrow
Q.58 Which one of the following options is the closest in meaning to the word given below?
Latitude
(A) Eligibility (B) Freedom (C) Coercion (D) Meticulousness
Q.59 Choose the most appropriate word from the options given below to complete the following sentence:
Given the seriousness of the situation that he had to face, his_was impressive.
(A) beggary (B) nomenclature (C) jealousy (D) nonchalance
Q.60 Choose the most appropriate alternative from the options given below to complete the following sentence:
If the tired soldier wanted to lie down, he ___ the mattress out on the balcony.
(A) should take
(B) shall take
(C) should have taken
(D) will have taken
Q. 61 - Q. 65 carry two marks each.
Q.61 One of the legacies of the Roman legions was discipline. In the legions, military law prevailed and discipline was brutal. Discipline on the battlefield kept units obedient, intact and fighting, even when the odds and conditions were against them.
Which one of the following statements best sums up the meaning of the above passage?
(A) Thorough regimentation was the main reason for the efficiency of the Roman legions even in adverse circumstances.
(B) The legions were treated inhumanly as if the men were animals.
(C) Discipline was the armies inheritance from their seniors.
(D) The harsh discipline to which the legions were subjected to led to the odds and conditions being against them.
Q.62 A and B are friends. They decide to meet between 1 PM and 2 PM on a given day. There is a condition that whoever arrives first will not wait for the other for more than 15 minutes. The probability that they will meet on that day is
(A) 1/4 (B) 1/16 (C) 7/16 (D) 9/16
Q.63 The data given in the following table summarizes the monthly budget of an average household.
|
Category |
Amount (Rs.) |
|
Food |
4000 |
|
Clothing |
1200 |
|
Rent |
2000 |
|
Savings |
1500 |
|
Other expenses |
1800 |
The approximate percentage of the monthly budget NOT spent on savings is (A) 10% (B) 14% (C) 81% (D) 86%
Q.64 There are eight bags of rice looking alike, seven of which have equal weight and one is slightly heavier. The weighing balance is of unlimited capacity. Using this balance, the minimum number of weighings required to identify the heavier bag is
(A) 2 (B) 3 (C) 4 (D) 8
Q.65 Raju has 14 currency notes in his pocket consisting of only Rs. 20 notes and Rs. 10 notes. The total money value of the notes is Rs. 230. The number of Rs. 10 notes that Raju has is
(A) 5 (B) 6 (C) 9 (D) 10
CY 20/20
GATE 2012 - Answer Key - Paper : CY
|
|
|
Attachment: |
| Earning: Approval pending. |
