Indian Institute of Technology Madras (IIT-M) 2012 GATE Life science with Answer Key - Question Paper
2012 XL
Duration: Three Hours Maximum Marks: 100
Read the following instructions carefully.
1. Do not open the seal of the Question Booklet until you are asked to do so by the invigilator.
2. Take out the Optical Response Sheet (ORS) from this Question Booklet without breaking the seal and read the instructions printed on the ORS carefully.
3. On the right half of the ORS, using ONLY a black ink ball point pen, (i) darken the bubble corresponding to your test paper code and the appropriate bubble under each digit of your registration number and (ii) write your registration number, your name and name of the examination centre and put your signature at the specified location.
4. This Question Booklet contains 28 pages including blank pages for rough work. After you are permitted to open the seal, please check all pages and report discrepancies, if any, to the invigilator.
5. There are a total of 65 questions carrying 100 marks. All these questions are of objective type. Each question has only one correct answer. Questions must be answered on the left hand side of the ORS by darkening the appropriate bubble (marked A, B, C, D) using ONLY a black ink ball point pen against the question number. For each question darken the bubble of the correct answer. More than one answer bubbled against a question will be treated as an incorrect response.
6. Since bubbles darkened by the black ink ball point pen cannot be erased, candidates should darken the bubbles in the ORS very carefully.
7. This Question Booklet contains Seven sections: GA (General Aptitude), H (Chemistry), I (Biochemistry), J (Botany), K (Microbiology), L (Zoology) and M (Food Technology).
8. Section GA (General Aptitude) and Section H (Chemistry) are compulsory. Attempt any two optional sections I through M. Using a black ink ball point pen, mark the sections you have chosen by darkening the appropriate bubbles provided on the left hand side of the ORS. Also, write the codes of the optional sections in the boxes provided. In case the candidate does not bubble section codes corresponding to Optional Section-1 or Optional Section-2 or both, the corresponding sections will NOT be evaluated.
9. Questions Q.1 - Q.10 belong to Section GA (General Aptitude) and carry a total of 15 marks. Questions Q.1 - Q.5 carry 1 mark each, and questions Q.6 - Q.10 carry 2 marks each.
10. There are 15 questions carrying 25 marks in Section H (Chemistry), which is compulsory. Questions Q1-Q5 carry 1 mark each and questions Q.6-Q.15 carry 2 marks each. The 2 marks questions include one pair of common data questions and one pair of linked answer questions. The answer to the second question of the linked answer questions depends on the answer to the first question of the pair. If the first question in the linked pair is wrongly answered or is unattempted, then the answer to the second question in the pair will not be evaluated.
11. Each of the other sections (Sections I through M) contains 20 questions carrying 30 marks. Questions Q.1-Q.10 carry 1 mark each and questions Q.11-Q.20 carry 2 marks each.
12. Unattempted questions will result in zero mark and wrong answers will result in NEGATIVE marks. For all 1 mark questions, % mark will be deducted for each wrong answer. For all 2 marks questions, 2A mark will be deducted for each wrong answer. However, in the case of the linked answer question pair, there will be negative marks only for wrong answer to the first question and no negative marks for wrong answer to the second question.
13. Calculator is allowed whereas charts, graph sheets or tables are NOT allowed in the examination hall.
14. Before the start of the examination, write your name and registration number in the space provided below using a black ink ball point pen.
|
Name | ||||||||
|
Registration Number |
XL | |||||||
General Aptitude (GA) Questions (Compulsory) Q. 1 - Q. 5 carry one mark each.
Q.1 If (1.001)1259 = 3.52 and (1.001)2062 = 7.85, then (1.001)3321 =
(A) 2.23 (B) 4.33 (C) 11.37 (D) 27.64
Q.2 One of the parts (A, B, C, D) in the sentence given below contains an ERROR. Which one of the following is INCORRECT?
I requested that he should be given the driving test today instead of tomorrow.
(A) requested that
(B) should be given
(C) the driving test
(D) instead of tomorrow
Q.3 Which one of the following options is the closest in meaning to the word given below?
Latitude
(A) Eligibility (B) Freedom (C) Coercion (D) Meticulousness
Q.4 Choose the most appropriate word from the options given below to complete the following sentence:
Given the seriousness of the situation that he had to face, his ___ was impressive.
(A) beggary (B) nomenclature (C) jealousy (D) nonchalance
Q.5 Choose the most appropriate alternative from the options given below to complete the following sentence:
If the tired soldier wanted to lie down, he ___ the mattress out on the balcony.
(A) should take
(B) shall take
(C) should have taken
(D) will have taken
Q. 6 - Q. 10 carry two marks each.
Q.6 One of the legacies of the Roman legions was discipline. In the legions, military law prevailed and discipline was brutal. Discipline on the battlefield kept units obedient, intact and fighting, even when the odds and conditions were against them.
Which one of the following statements best sums up the meaning of the above passage?
(A) Thorough regimentation was the main reason for the efficiency of the Roman legions even in adverse circumstances.
(B) The legions were treated inhumanly as if the men were animals.
(C) Discipline was the armies inheritance from their seniors.
(D) The harsh discipline to which the legions were subjected to led to the odds and conditions being against them.
Q.7 A and B are friends. They decide to meet between 1 PM and 2 PM on a given day. There is a condition that whoever arrives first will not wait for the other for more than 15 minutes. The probability that they will meet on that day is
(A) 1/4 (B) 1/16 (C) 7/16 (D) 9/16
Q.8 The data given in the following table summarizes the monthly budget of an average household.
|
Category |
Amount (Rs.) |
|
Food |
4000 |
|
Clothing |
1200 |
|
Rent |
2000 |
|
Savings |
1500 |
|
Other expenses |
1800 |
The approximate percentage of the monthly budget NOT spent on savings is (A) 10% (B) 14% (C) 81% (D) 86%
Q.9 There are eight bags of rice looking alike, seven of which have equal weight and one is slightly heavier. The weighing balance is of unlimited capacity. Using this balance, the minimum number of weighings required to identify the heavier bag is
(A) 2 (B) 3 (C) 4 (D) 8
Q.10 Raju has 14 currency notes in his pocket consisting of only Rs. 20 notes and Rs. 10 notes. The total money value of the notes is Rs. 230. The number of Rs. 10 notes that Raju has is
(A) 5 (B) 6 (C) 9 (D) 10
H : CHEMISTRY (Compulsory) Q. 1 - Q. 5 carry one mark each.
Q.1 Among the following, the most reactive diene in the Diels-Alder reaction is (A) (B)
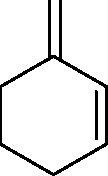
(D)
(C)
Q.2 The molecule that does NOT absorb the microwave radiation is (A) CO2 (B) H2O
(D) NO
(C) CO
Q.3 The hybridization of atomic orbitals of sulphur in SF4 is
(A) dsp2 (B) sp3d2
(C) sp3d
(D) sp3
+ - 2+ 3+
Q.4 The ionic size of Na , F , Mg and Al varies as
|
3+ 2+ + - (A) Al3 > Mg2 > Na > F 3+ 2+ - + (C) Al3 > Mg2 > F > Na |
- + 2+ 3+ (B) F > Na > Mg2 > Al3 + - 2+ 3+ (D) Na > F > Mg2 > Al3 |
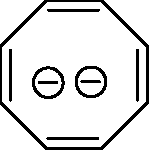
Q.5 The non-aromatic compound/ion is (A)
(B)
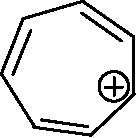
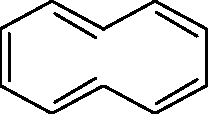
(D)
(C)
Q. 6 - Q. 15 carry two marks each.
Q.6 As predicted by MO theory, the bond order and magnetic nature of NO+ are
(A) three and paramagnetic (B) two and diamagnetic
(C) two and paramagnetic (D) three and diamagnetic
-14 -13
Q.7 The value of ionic product of water changes with temperature. It is 1 x 10 at 25 oC and 1 x 10 at 60 C. The CORRECT statement with respect to AH and AS is
|
(A) AH is negative and AS is negative (C) AH is positive and AS is negative |
(B) AH is positive and AS is zero (D) AH is negative and AS is positive |
Q.8 10 micrograms of the enzyme carbonic anhydrase (molecular weight = 30,000 g/mole) removes 300 milligrams of carbon dioxide per minute from the cells. The turnover number of the enzyme is
(A) 20 min-1
(B) 2 x 107 min-1 (D) 7.2 x 1010 min-1
(C) 2 x 1010 min-1
Q.9 The iodide which reacts most slowly with cyanide ion as a nucleophile in a SN2 reaction is
(A)
(B)
CHI
ch3ch2ch2ch2i
(D)
(C)
(CH3)2CH-I
Q.10 The value of Ka of acetic acid is 1.7 x 10 5 mol/dm3. The pH of a buffer solution prepared by mixing 100 ml of 0.1 M acetic acid with a solution of 100 ml of 0.2 M sodium acetate is
(A) 4.1 (C) 5.1
(B) 4.5 (D) 5.5
Q.11 The value of standard half cell potential of Cu+2, Cu couple (E0Cu(+2), Cu) is 0.34 V. A wire of pure copper is immersed into a solution of copper nitrate. If the measured cell potential against standard hydrogen electrode at 298 K is 0.24 V, the molar concentration of copper nitrate is {Assume activity of Cu+2 = [Cu+2]}.
(A) 4.1 x 10-4 M (B) 2.0 x 10-2 M
(C) 3.4 x 10-2 M (D) 1.8 x 10-1 M
Common Data for Questions 12 and 13:
[FeCl4]2- (I), [CoCl4]2- (II) and [NiCl4]2- (III) are paramagnetic tetrahedral complexes. Q.12 The order of values of crystal field stabilization energy is
(A) I > III > II (C) I > II > III
(B) III > I > II (D) II > III > I
Q.13 The order of values of spin only magnetic moment is
(A) III > II > I (C) II > I > III
(B) III > I > II (D) I > II > III
Statement for Linked Answer Questions 14 and 15:
Bromine water is decolourised upon reaction with ()-3-hexene.
Q.14 It is due to
(A) electrophilic addition of bromine to C=C (B) nucleophilic addition of bromine to C=C
(C) electrophilic allylic bromination (D) nucleophilic allylic bromination
Q.15 The structure of the product obtained is
(A)
(B)
Br
|
Br |
 |
|
Br |
Br
(D)
(C)
|
Br |
 |
|
Br |
|
Br |
 |
|
Br |
I : BIOCHEMISTRY Q. 1 - Q. 10 carry one mark each.
Q.1 Four proteins (P1, P2, P3 and P4) have 17, 10, 21 and 14 percent hydrophobic amino-acids respectively. The order of precipitation of these proteins using ammonium sulphate will be
(A) P3, P1, P4, P2 (B) P3, P1, P2, P4 (C) P2, P4, P3, P1 (D) P2, P4, P1, P3
Q.2 Which one of the following pairs of amino-acids in the protein has high propensity to take up the a-helix conformation?
(A) Gly-Asp (B) Pro-His (C) Gly-Pro (D) Ala-Arg
Q.3 Which one of the following closely defines Molten Globule state of a protein?
(A) State with high degree of secondary structure and loss of tertiary structure
(B) State with complete loss of secondary structure
(C) Completely unfolded state
(D) Loss of quaternary structure
Q.4 Which one of the following amino-acids has highest fluorescence quantum yield (O) in aqueous solution?
(A) Tyrosine (B) Tryptophan (C) Phenylalanine (D) Histidine
Q.5 Which one of the following compounds does NOT block electron transport?
(A) Cyanide (B) Rotenone (C) Oligomycin (D) Antimycin A
Q.6 The pair of amino-acids which does NOT undergo post-translational modification is (A) Asn-His (B) Tyr-Ser (C) Asn-Ser (D) Ala-Gly
Q.7 Match the hormones in Group I with their metabolic precursor in Group II Group I Group II
P. 17-p estradiol 1. Arachidonic acid
Q. Thromboxane A2 2. Tyrosine
R. Epinephrine 3. p-carotene
S. Retinoic acid 4. Cholesterol
(A) P-4, Q-2, R-3, S-1 (B) P-1, Q-3, R-2, S-4
(C) P-4, Q-1, R-2, S-3 (D) P-1, Q-2, R-4, S-3
Q.8 Upon stimulation of a eukaryotic cell, the intracellular calcium (Ca2+) is released from
(A) Endoplasmic reticulum (B) Nucleus
(C) Peroxisome (D) Mitochondria
Q.9 Leguminous plants maintain a very low concentration of free oxygen in their root nodules because
(A) the nitrogen fixing bacteria living in the root nodules are anaerobic
(B) of binding of oxygen to leghemoglobin
(C) reductase enzyme of the nitrogenase complex helps in removal of O2
(D) nitrogenase enzyme of the nitrogenase complex helps in removal of O2
Q.10 The membrane of mature B cells have
(A) both IgG and IgM (B) both IgG and IgD
(C) both IgM and IgE (D) both IgM and IgD
Q. 11 - Q. 20 carry two marks each.
Q.11 An amino-acid has one proton donating group in the side chain (R). The pKCOOH, pKNH2 and pKR values for this amino-acid are 2.19, 9.67 and 4.25, respectively. Which one of the following statements about this amino-acid is CORRECT?
(A) Majority of the molecules will have a net charge of -1 at pH of 7.0
(B) Majority of the molecules will have a net charge of 0 at pH of 4.25
(C) All the molecules will have a deprotonated R group at pH of 3.22
(D) During titration with a strong base, deprotonation will start with the R group
Q.12 Which one of the following bacterial toxins does NOT have ADP-ribosyl transferase activity?
(A) Pertussis toxin (B) Diphtheria toxin
(C) Pseudomonas Exotoxin A (D) S. aureus a-toxin
Q.13 The CORRECT pair of amino-acid sequence and the corresponding target organelle is
(A) KDEL - Golgi (B) K-K/R-X-K/R - Lysosome
(C) SKL - Peroxisome (D) NPVY - Endoplasmic reticulum
Q.14 p-oxidation of a 16 carbon fatty acid and a 17 carbon fatty acid leads to formation of
(A) (8 Acetyl CoA) and (8 Acetyl CoA + CO2), respectively
(B) (5 Propionyl CoA + 1 CO2) and (5 Propionyl CoA +1 Acetyl CoA), respectively
(C) (5 Propionyl CoA + 1 CO2) and (5 Propionyl CoA + 2 CO2), respectively
(D) (8 Acetyl CoA) and (7 Acetyl CoA + 1 Propionyl CoA), respectively
Q.15 Pick the correctly matched pairs.
P. Immature B cells Terminal deoxynucleotidyl transferase
Q. Activated B cells Class switching
R. Pre B cells Surrogate light chain
S. Mature B cells Recombination activating gene 1
(A) P and R (B) Q and R
(C) Q and S (D) Q and P
Q.16 The actual free energy change of a given biochemical reaction carried out under standard conditions with 1 M initial concentration of each of the reactants and products will be
(A) equal to zero
(B) equal to standard free energy change for the reaction
(C) less than the standard free energy change for the reaction
(D) greater than the standard free energy change for the reaction
Q.17 Match the enzymes in Group I with their corresponding activity in Group II
P. Flippase 1. Catalyzes the movement of any phospholipid across the lipid bilayer
down its concentration gradient
Q. Floppase 2. Catalyzes the translocation of amino-phospholipids from the
extracellular to the inner leaflet
R. Lipase 3. Catalyzes the translocation of membrane phospholipids from cytosolic to
the extracellular leaflet
S. Scramblase 4. Degradation of phospholipids from the lipid bilayer including the inner
and outer leaflets
(A) P-2, Q-3, R-4, S-1 (C) P-4, Q-1, R-2, S-3
(B) P-1, Q-2, R-3, S-4 (D) P-3, Q-2, R-4, S-1
Q.18 Match the antibiotics in Group I with their mechanism of action in Group II
|
Group I |
Group II | |
|
P. Tetracyclines |
1. |
Inhibits bacterial protein synthesis by blocking peptidyl transfer |
|
Q. Chloramphenicol |
2. |
Inhibits bacterial protein synthesis by blocking the A-site on the ribosome |
|
R. Cycloheximide |
3. |
Misreads the genetic code and inhibits initiation of protein synthesis |
|
S. Streptomycin |
4. |
Inhibits protein synthesis by blocking peptidyl transferase on 80S ribosome |
|
(A) P-2, Q-1, R-3, S-4 |
(B) P-2, Q-1, R-4, S-3 | |
|
(C) P-4, Q-3, R-1, S-2 |
(D) P-3, Q-4, R-2, S-1 | |
Q.19 The kinetics of an enzyme in the presence (+I) or absence (-I) of a reversible inhibitor is described in the following graph.
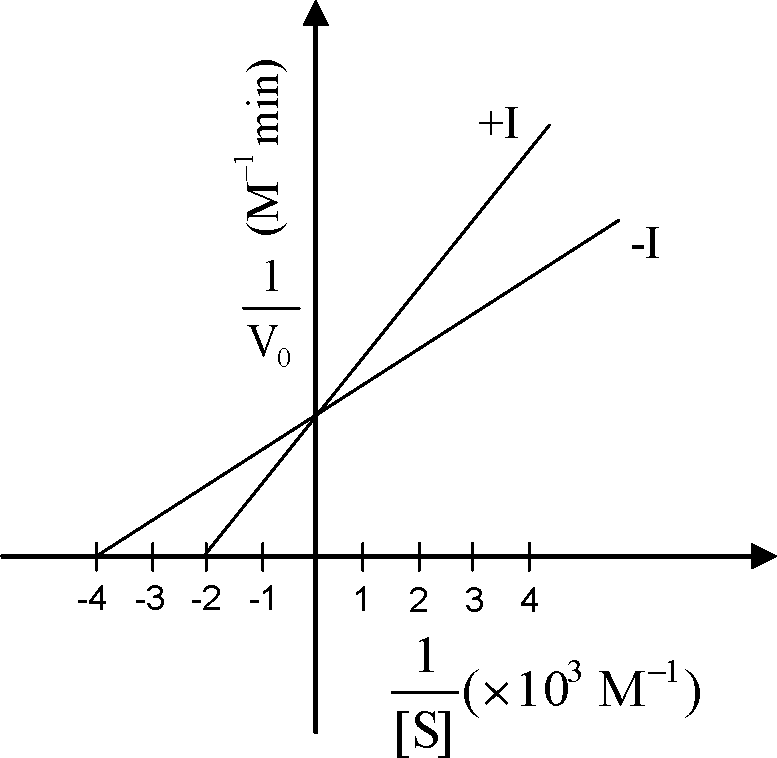
If concentration of the reversible inhibitor in +I experiment was equal to 3.0 x 10 3 M, then the dissociation constant for the enzyme-inhibitor complex is
(A) 1 x 10-3 M (B) 2 x 10-3 M (C) 3 x 10-3 M (D) 4 x 10-3 M
Q.20 The figure below is a schematic of a linear double stranded DNA containing the indicated restriction sites. The DNA was completely digested with PvuII and EcoRI. The products were purified and added to an appropriately buffered reaction mixture containing dNTP mix, a-32P dATP, and Klenow fragment of E. coli DNA polymerase I. The Klenow reaction products were analyzed by gel electrophoresis and autoradiography. Which of the following products depicts the expected result?
Pvu\\ CAGCTG GTCGAC
EcoRI
Pvu\\
3 kb
1 kb
2 kb
GAATTC
EcoRI
CTTAAG
|
5 kb - |
5 kb - |
5 kb - |
5 kb - | ||||
|
4 kb -3 kb- |
4 kb - (B) 3 kb- |
4 kb - (C) 3 kb - |
4 kb - (D) 3 kb - | ||||
|
2 kb - |
2 kb - |
2 kb - |
- |
2 kb - | |||
|
1 kb- |
1 kb- |
- |
1 kb- |
1 kb- |
J:BOTANY Q. 10 carry one mark each.
The swollen base of a petiole is known as
2012 Q. 1
Q.1
Q.2
Q.3
Q.4
Q.5
Q.6
Q7
Q.8
Q9
Q.10
(A) Ligule (B) Hastule (C) Pulvinus (D) Stipule
An estimate of phylogenetic relationships among the taxa is commonly represented in the form of a (A) Cladogram (B) Idiogram (C) Phenogram (D) Dendrogram
Parenchyma cells associated with sieve tube members are called
(A) Albuminous cells (B) Companion cells (C) Bulliform cells (D) Subsidiary cells
Cytoplasmic male sterility via the chloroplast genome can be induced by the expression of Pha A gene encoding
(A) p-Ketothiolase
(B) Acetoacetyl CoA carboxylase
(C) Acetoacetyl CoA reductase
(D) PHB synthase
The number of nucleosomes present in a 30 nm solenoid structure of a chromatin is (A) 2 (B) 4 (C) 6 (D) 8
Which one of the following performs normal C3 photosynthesis when water is available, but switches to crassulacean acid metabolism (CAM) during salt or drought stress?
(A) Mesembryanthemum crystallinum (B) Cynodon dactylon
(C) Eleucine coracana (D) Hordeum vulgare
Which one of the following is a free-living photosynthetic nitrogen fixer?
(A) Frankia (B) Clostridium (C) Rhodospirillum (D) Rhizobium
Carbon dioxide and other greenhouse gases act by
(A) destroying ozone in the stratosphere
(B) trapping heat in the earths atmosphere
(C) allowing more visible light to reach the earths surface
(D) reducing the amount of radiant energy which reaches the surface of the earth
Which of the following best represents the flow of energy through an ecosystem?
(A) Producers Primary consumers Secondary consumers
(B) Sun Producers Secondary consumers Primary consumers
(C) Sun Producers Primary consumers Secondary consumers
(D) Secondary consumers Primary consumers Producers Sun
Which one of the following drugs is obtained from the capsule of Papaver somniferum?
(A) Papain (B) Codeine (C) Digoxin (D) Bromelain
Q. 11 - Q. 20 carry two marks each.
Q.11 Which of the following statements are TRUE for plant growth regulators?
P. The release of cellulase and polygalacturonase into the cell wall is promoted by abscissic acid Q. The early biosynthetic steps of gibberellic acid, up to the formation of ent-kaurene take place in the plastid
R. The naturally occurring zeatin belongs to the aromatic group of cytokinins
S. Induction of protease inhibitors as a result of wounding and pathogen attack is activated by jasmonic acid
(A) P, R (B) Q, S (C) Q, R (D) P, Q
Q.12 Which of the following statements are TRUE for the transposable elements?
P. Barbara McClintock discovered the autonomous and non-autonomous transposable elements in Maize
Q. Variations in flower pigmentation in Antirhinum are due to the presence of transposable elements Ac and Ds
R. The Ac transposable element is 4563 bp long and has an 11 bp inverted repeats
S. Ds produces the transposase and mobilize the Ac elements
(A) Q, S (B) P, Q (C) P, R (D) R, S
Q.13 Match the recombinant proteins produced through molecular farming with their applications.
|
Recombinant proteins Applications | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Q.14 Which of the following statements are CORRECT for somatic cell hybridization?
P. For fusion of protoplast, dimethylsulfoxide (DMSO) is used as a fusogen Q. The enzyme Cellulase Onozuka used for protoplast isolation is sourced from Trichoderma viride
R. The first report of somatic hybrid plants resulted from the fusion of protoplasts of Nicotiana glauca and N. tabacum
S. Viability of isolated protoplasts can be determined by Evans blue staining
(A) P, Q (B) Q, S (C) R, S (D) Q, R
Q.15 Which of the following statements are TRUE on transgene approach?
P. T-DNA integration occurs mainly through non-homologous recombination Q. The Gateway cloning depends on recombination technology as opposed to standard uses of restriction enzymes and DNA ligase R. The localization of p-glucuronidase (GUS) activity as a result of expression of GUS reporter gene can be visualized in a histochemical assay using the X-gal S. The green fluorescent protein gene (GFP) is isolated from the bacterium Photinus pyralis
(A) P, Q (B) Q, R (C) P, S (D) R, S
Q.16 Identify the free radicals (marked as ?) in sequence from the inter-conversion of reactive oxygen species as shown below.
O2 H2O2 H2O
p. o; -
Q. OH -R. HO 2S. 'O2
(A) P, Q (B) R, Q (C) P, R (D) Q, S
Q.17 With respect to adhesion and cohesion of stamens, identify the INCORRECT statements.
P. Adnation of stamens to petals is described as epiphyllous stamens Q. In Calotropis, stamens and carpels are united to form gynostegium R. In syngenesious stamens, filaments are united to form a bundle while the anthers are free S. Synandrous stamens found in Cucurbita represent the union of filaments as well as anthers
(A) P, Q (B) P, R (C) Q, S (D) Q, R
Q.18 Identify the CORRECT statements in plant secondary metabolism.
P. Tropane alkaloids in Atropa belladonna are synthesized from tyrosine
Q. Antioxidative food ingredient rosmarinic acid is obtained from cell suspension cultures of
Coleus blumei
R. Thiophenes are produced from hairy root cultures of Tagetes patula S. Cyanidin, the principal anthocyanin responsible for red color in Rosa hybrida is produced from cinnamaldehyde
(A) P, S (B) R, S (C) P, Q (D) Q, R
Q.19 Which of the following statements are TRUE for respiration?
P. The conversion of one molecule of pyruvate to three molecules of CO2 generates four molecules of NADH Q. Fructose 6-phospate is the principal substrate for glycolysis
R. The oxidation of glucose 6-phosphate to 6-phosphogluconate is the first step in the oxidative pentose phosphate pathway S. The mitochondrial alternative oxidase provides an alternative pathway for transfer of electrons from ubiquinone to oxygen utilizing proton pumping complex of the respiratory chain
(A) P, R (B) P, S (C) Q, R (D) Q, S
Q.20 Match the name of the disease with the causal organism. Disease Causal organism
|
P. Black rot of sugarcane Q. Stem rot of jute R. Tikka disease of groundnut 1. Cercospora personata 2. Macrophomina phaseolina 3. 4. S. Crown gall of grapes |
Ceratocystis adiposa Synchytrium endobioticum 5. Agrobacterium tumefaciens 6. Colletotrichum corchorum |
|
|
K : MICROBIOLOGY Q. 1 - Q. 10 carry one mark each.
Q.1 Which ONE of the following components is NOT an electron acceptor during anaerobic respiration? (A) Lactate (B) Carbonate (C) Nitrate (D) Sulphate
Q.2 Bergeys Manual of Systematic Bacteriology groups bacteria into species according to their
(A) nutritional requirement
(B) phylogenetic relationships
(C) pathogenic properties
(D) morphology
Q.3 An auxotrophic mutant arises spontaneously in a wild type E.coli culture growing in a rich medium. Which ONE of the following techniques will ensure the isolation of the auxotrophic mutant?
(A) Replica plating (B) Streaking for single colonies
(C) Pour plating method (D) Direct microscopic observation
Q.4 Which ONE of the following mutants is used to carry out genetic analysis to determine the function of an essential gene?
(A) Knock out mutant (B) Deletion mutant
(C) Insertion mutant (D) Temperature sensitive mutant
Q.5 An E.coli mutant constitutive for the lac operon was mated with a wild type strain. The merodiploid thus obtained was inducible by lactose. This observation indicates that the original mutation is
(A) dominant (B) trans-dominant (C) recessive (D) cis-dominant
Q.6 A rich medium is inoculated with a bacterium that divides every 30 minutes. The number of bacteria at the end of 50 hours is
(A) 2 x 1010 (B) 2 x 1020 (C) 1 x 1050 (D) 1 x 2100
Q.7 Which ONE of the following statements about E.coli is NOT true ?
(A) E.coli was the first disease- causing bacterium identified by Robert Koch (BJ E.coli is part of the normal microbiota of humans
(C) Certain E.coli strains can cause bloody diarrohea (DJ E.coli is beneficial to human
Q.8 Antibody coated pathogens are recognized by effector cells through
(A) CD4 receptor (B) FC receptor (C) CD8 receptor (D) IFN gamma receptor
Q.9 Match the disease in Group I with their corresponding organism in Group II
Group II
Group I
P. African sleeping sickness Q. Rocky mountain spotted fever R. Mumps
I. Rubulavirus
II. Trypanosoma brucei
III. Wuchereria bancrofti
IV. Rickettsia rickettsii
V. Leishmania donovani
S. Filariasis
(A) P-III, Q-V, R-II, S- I
(B) P-II, Q-I, R-III, S- IV
(C) P-II, Q-IV, R-I, S- III
(D) P-I, Q-V, R-II, S- IV
Q.10 Select the technique most appropriate to demonstrate that lactose induces the synthesis of P-galactosidase enzyme.
(A) Northern Blot
(B) Western Blot
(C) Quantitative PCR
(D) Southern Blot
Q. 11 - Q. 20 carry two marks each.
Q.11 Frederick Griffith used smooth (S) and rough (R) strains of Streptococcus pneumoniae in his
classical experiment that showed DNA might be the genetic element. Which ONE of the following observations gave the clue for this discovery?
(A) R strain became S strain when mixed with heat killed S strain
(B) R strain remained R strain when mixed with heat killed S strain
(C) S strain became R strain when mixed with heat killed R strain
(D) R strain became S strain when mixed with live S strain
Q.12 Entry of A phage lysogen to lytic phase is triggered by
(A) mutation in the A genome
(B) loss of co-operativity in binding of A repressor
(C) increase in the A repressor concentration
(D) decrease in recA function
Q.13 Match the Phylum in Group I with their characteristic motility appendage listed in Group II
Group II
Group I
P. Archaezoa Q. Amoebozoa R. Ciliophora
I. Flagella
II. Fimbriae
III. Pseudopods
IV. Cilia
V. Pili
S. Apicomplexa
(A) P-V, Q-II, R-IV, S-IV
(B) P-II, Q-I, R-IV, S-III
(C) P-I, Q-III, R-IV, S-I
(D) P-III, Q-II, R-IV, S-V
Q.14 Ten bacteria were inoculated into a rich medium. If at the end of ten hours the total number of cells is 104, then the number of elapsed generations and the generation time respectively is
(A) 10, 120 minutes (B) 10, 60 minutes (C) 20, 30 minutes (D) 40, 15 minutes
Q.15 The first step in the replication of a virus with the reverse transcriptase deals with the synthesis of
(A) complementary strand of RNA
(B) double stranded RNA
(C) complementary strand of DNA
(D) double stranded DNA
Q.16 An E.coli mutant defective for an enzyme is unable to grow on acetate but grows on glycerol as the sole carbon source. Which ONE of the following enzymes is likely to be defective in this mutant?
(A) Isocitrate dehydrogenase (B) Glyceraldehyde 3-phospahte dehydrogenase
(C) Pyruvate dehydrogenase (D) Isocitrate lyase
Q.17 Which one of the following pairs of bacterial species fixes atmospheric Nitrogen?
(A) Clostridia and Rhizobia
(B) Clostridia and Lactobacillus
(C) Rhizobia and Enterococcus
(D) Actinomycetes and Mycoplasma
Q.18 Nalidixic acid inhibits gyrase activity. Resistance to this antibiotic arises mainly due to
(A) nonsense mutation in the gyrase gene (B) deletion mutation in the gyrase gene (C) missense mutation in the gyrase gene (D) degradation of the gyrase gene product
Q.19 Transformation of normal cyanobacterial cells into heterocysts involves
(A) synthesis of nitrogenase and retention of photosystem I
(B) synthesis of nitrogenase and loss of photosystem I
(C) loss of nitrogenase but retention of photosystem I
(D) loss of both nitrogenase and photosystem I
Q.20 Methane belched (eructation) out by cattle arises from the carbon dioxide produced
(A) during normal respiration
(B) oxidation of food stuff occurring in mitochondria
(C) lactic acid fermentation occurring in muscles
(D) bacterial fermentation occurring in the gut
Q.2 A swimmer is preparing to swim non-stop across the English channel (a distance of 34 kilometers). Consumption of which of the following category of food/s should the swimmer increase to accomplish this feat?
Q. 1 - Q. 10 carry one mark each.
Q.1 Trees in the equatorial region of earth supply oxygen into the atmosphere that sustains species living in distant polar regions. This relationship is called as
(A) mutualism (C) commensalism
(B) symbiosis (D) parasitism
(A) Proteins
(B) Fats
(D) Carbohydrates
(C) Proteins and Carbohydrates
Q.3 Some species of beetles and fishes can survive at sub freezing temperature. They accomplish this by maintaining cellular integrity using one of the following mechanisms.
(A) Expressing anti-freeze proteins
(B) Accumulating fats
(C) Increasing accumulation of complex polyols
(D) Reducing the availability of total water in the body
Q.4 Zygotic genes required for the formation of a group of adjacent segment in the developing Drosophila embryo is called
(A) Maternal gene (B) Pair rule gene (C) Homeotic gene (D) Gap gene
Q.5 A typical receptor senses extracellular stimuli by virtue of its localization on plasma membrane. The receptor to which of the following ligands is an exception to this rule?
(A) y-amino butyric acid (B) Acetylcholine
(C) Estrogen (D) Luteinizing hormone
Q.6 The relationship between genes and enzymes was first suggested by the discovery of
(A) in-born errors of metabolism in human
(B) sexual phenotype in insects
(C) metabolic pathways in fungi
(D) gene regulation in bacteria
Q.7 The blind spot in the retina is blind because of which of the following reasons?
(A) It is the region where the optical nerve leaves the retina.
(B) The opsin is not expressed in this region.
(C) It lies in the shadow of pupil.
(D) It is the junction between rods and cones.
Q.8 Lamprey, a jawless fish, belongs to which one of the following Classes?
(A) Myxini (B) Cephalaspilomorphi
(C) Conodonta (D) Anaspida
Q.9 Chorionic gonadotropin plays an important role in the establishment and maintenance of pregnancy and is synthesized in the placenta of
(A) Cattle
(B) Pigs
(C) Mice
(D) Human
Q.10 Glucose and hexanoic acid, each having six carbon atoms can undergo complete biological oxidation. In terms of net ATP generation, which of the following statements is CORRECT?
(A) Glucose produces more ATP than hexanoic acid
(B) Only glucose can generate ATP
(C) Both glucose and hexanoic acid produce same amount of ATP
(D) Hexanoic acid produces more ATP than glucose
Q. 11 - Q. 20 carry two marks each.
Q.11 Match the following evolutionary biologists with their respective theory
i) Neutral theory of molecular evolution
I) August Weisman
II) Jean-Baptiste Lamark
III) Amotz Zahavi
IV) Motoo Kimura
(A) I-ii, II-iv, III-i, IV-iii (C) I-iii, II-iv, III-i, IV-ii
ii) Handicap principle
iii) Germ plasm theory
iv) Inheritance of acquired characteristics
(B) I-iii, II-iv, III-ii, IV-i
(D) I-iii, II-i, III-iv, IV-ii
Q.12 A female cat with a mutant phenotype was bred with a wild-type male cat. All progeny (4 males and 4 females) show the mutant phenotype. On the other hand, all progeny (4 males and 4 females) from the reciprocal cross between a mutant male and a wild-type female show the wild-type phenotype. Which of the following explain the inheritance pattern of the mutation?
(A) Recessive (B) Linked inheritance
(C) Mitochondrial inheritance (D) Autosomal inheritance
Q.13 If all the nucleotides have equal probability of occurrence in a 4 Mbp long DNA sequence, then how many times will the site of EcoRI, restriction endonuclease occur?
(A) 976 (B) 46 (C) 64 (D) 1000
Q.14 Seasonally breeding animals and birds measure the day length, i.e. photoperiod and use these measurements as predictive information to prepare themselves for breeding. Besides melatonin, which of the following hormones is involved in this biological process?
(A) Gonadotropin releasing hormone (B) Growth hormone
(C) Thyroxine (D) Adrenocorticotropic hormone
Q.15 Red-Green color blindness is an X-linked recessive disorder. In a population which is in the Hardy-Weinberg equilibrium, the incidence of occurrence of this in males is 1:1000. What will be the expected incidence of affected homozygous females?
(A) 1 in 1002000 (B) 1 in 2000000 (C) 1 in 1001000 (D) 1 in 1000000
Q.16 Golgi apparatus is also termed as cellular post office, since it packages and transports cellular proteins across various organelles and outside the cell. In general, the Golgi is perinuclear in location and is closely associated with the endoplasmic reticulum. A chemical compound, Monensin inhibits all trafficking from Golgi. If Golgi is visualized by immunofluorescence microscopy after treatment with this compound, the Golgi will be
(A) absent (B) normal (C) swollen (D) fragmented
Q.17 In an individual, three distinct proteins bind oxygen depending on the location and development stage. While hemoglobin is the major oxygen binding protein in adults, myoglobin is present in skeletal muscles and fetal hemoglobin is present in fetal stage only. The following graph shows the oxygen binding capacity of these proteins. The A, B and C plots represent oxygen binding capacity of
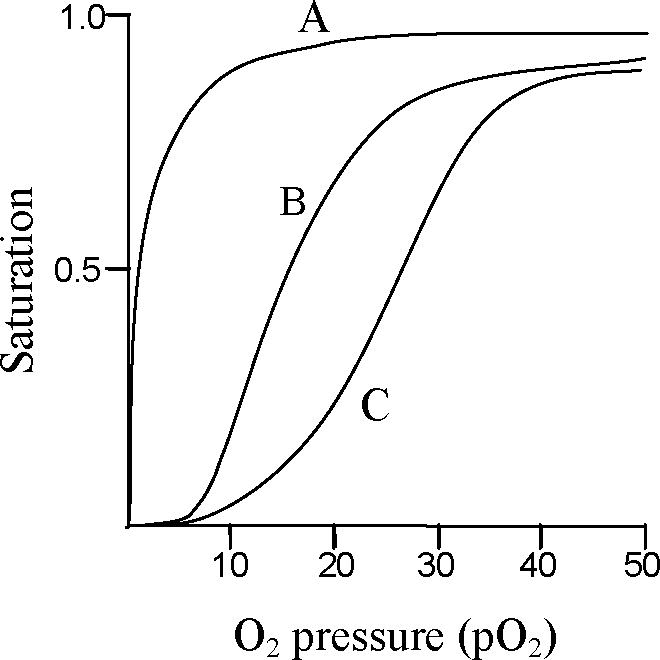
(A) hemoglobin, fetal hemoglobin and myoglobin, respectively
(B) fetal hemoglobin, hemoglobin and myoglobin, respectively
(C) hemoglobin, myoglobin and fetal hemoglobin, respectively
(D) myoglobin, fetal hemoglobin and hemoglobin, respectively
Q.18 A patient comes with symptoms of autonomic hemolysis. The diagnostic tests reveal that he has auto-antibodies to red blood cells (RBCs). Which one of the following mechanisms is the cause of this condition?
(A) Neutrophils release granzymes which lyse RBCs
(B) Complement is activated and membrane attack complex lyse RBCs
(C) Cytotoxic T-cells lyse RBCs
(D) Interleukin-2 binds to the receptor on RBCs
Q.19 The nerve impulse at the neuromuscular junction results in discharge of acetylcholine (Ach) from its vesicles into the synaptic cleft. Ach gets degraded by acetylcholine esterase and is present in which one of the following locations?
(A) Post synaptic membrane (B) Both pre and post - synaptic clefts
(C) Presynaptic membrane (D) Synaptic cleft
Q.20 Increasing estradiol (E2) hormone from ovarian follicles prior to ovulation has been hypothesized to play a critical role for induction of pheromones. These pheromones render females sexually receptive to males to facilitate mating. An investigator performs experiments in sheep in which females are gonadectomized, then treated with E2 or vehicle alone and allowed to breed. Which one of the findings listed below will validate the hypothesis that pheromones are induced by E2?
(A) Sexual receptivity is regained only in vehicle treated females.
(B) Sexual receptivity is regained only in E2 treated females
(C) Sexual receptivity was regained irrespective of E2 treatment
(D) Sexual receptivity is not regained by any treatment
M:FOOD TECHNOLOGY Q. 1 - Q. 10 carry one mark each.
Q.1 Among the following fatty acids, which group is known as essential fatty acids?
(A) 9,11 -Octadecadienoic and 9,11,13 -Octadecatrienoic
(B) 9,12-Octadecadienoic and 9,12,15-Octadecatrienoic
(C) 9-Octadecenoic and 9,11 -Octadecadienoic
(D) 9,11-Octadecadienoic and 9-Eicosenoic
Q.2 Cellulose, the structural polysaccharide of plant, is a polymer of
(A) p-D-Glucose
(B) a-D-Glucose
(C) p-D-Galactose
(D) a-D-Galcturonic acid
Q.3 The important role of carotenoids in the human diet is their ability to serve as precursors of
(A) Vitamin C (B) Vitamin D (C) Vitamin A (D) Vitamin K
Q.4 Which one of the following microorganisms is used in the preparation of bread?
(A) Candida utilis (B) Saccharomyces cerevisiae
(C) Saccharomyces cevarum (D) Aspergilus niger
Q.5 Which one of the microorganisms given below is NOT RESPONSIBLE for ropy or stringy fermentation of milk?
(A) Alcaligenes viscolactis
(B) Enterobacter aerogenes
(C) Streptococcus cremoris
(D) Streptococcus lactis
Q.6 A mild heat treatment of foods that destroys pathogens and extends its shelf life is called
(A) Baking (B) Blanching
(C) Sterilization (D) Pasteurization
Q.7 The most common and least expensive plastic film used for packaging of solid food materials is
(A) Polyethylene (B) Polystyrene
(C) Polypropylene (D) Polyvinylchloride
Q.8 Reassociation of amylose and formation of crystalline structure upon cooling of cooked starch solution is termed as
(A) Synersis (B) Gelatinization
(C) Retrogradation (D) Denaturation
Q.9 Thermal destruction of microorganisms follows a kinetics of
(A) Zero order (B) First order (C) Second order (D) Fractional order
Q.10 100 kg tomato juice containing 5% Total Solids (w/w) is concentrated to 25% Total Solids (w/w). The total amount of water removed from tomato juice in kg is
(A) 65 (B) 70 (C) 75 (D) 80
Q. 11 - Q. 20 carry two marks each.
Q.11 Which one of the following is NOT A CORRECT statement?
(A) Meatiness is the taste produced by compounds such as glutamate in products like cheese, soy sauce.
(B) Astringency is a dry mouth feel in the oral cavity that is most associated with phenolic compounds.
(C) Saltiness is a taste that is mainly produced by chloride ions.
(D) Sourness is related to acidity and is sensed by hydrogen ion channels in the human tongue.
Q.12 The following plot represents the Lineweaver-Burk equation of an enzymatic reaction both in the presence and the absence of inhibitor. Here, V is the velocity of reaction and S is the substrate concentration.
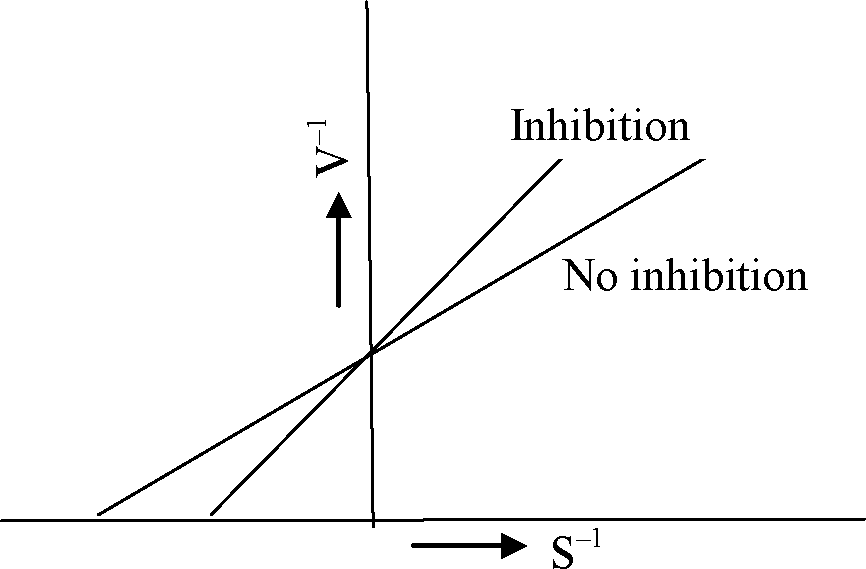
The nature of inhibition shown in the plot is
(A) Non-competitive
(B) Anti-competitive
(C) Competitive
(D) Mixed type
Q.13 Make the correct match of the food constituents in Group I with their nature given in Group II.
Group II
Group I
P) Ascorbic Acid Q) Phenyl alanine R) Dextrose S) Haemoglobin
(A) P-4, Q-3, R-1, S-2 (C) P-3, Q-4, R-2, S-1
1) Sugar
2) Chelate
3) Amino Acid
4) Antioxidant
(B) P-4, Q-1, R-3, S-2
(D) P-4, Q-2, R-1, S-3
Q.14 Make the correct match of the fermented food products in Group I with the microorganisms in
Group II.
Group I
P) Yoghurt Q) Cheese R) Sauerkraut S) Kefir
(A) P-1, Q-4, R-2, S-3 (C) P-3, Q-4, R-2, S-1
Group II
1) Lactobacillus acidophilus and Lactobacillus delbrueckii
2) Leuconostoc mesenteroides and Lactobacillus plantarum
3) Lactobacillus delbrueckii and Streptococcus thermophillus
4) Lactobacillus casei and Streptococcus thermophillus
(B) P-4, Q-3, R-1, S-2
Q.15
