Veer Narmad South Gujarat University 2011-1st Sem M.Sc Chemistry S-2604 : - 4 - Question Paper
S-2604
M. Sc. (Sem. I) Examination March / April - 2011 Chemistry : Paper - IV (Instrumental & Chemical Analysis)
Time : 3 Hours]
[Total Marks : 70
Instructions :
N Seat No.:
6silq<3i - Puunki(l faaim SuwiA u* <KH=fl. Fillup strictly the details of signs on your answer book.
Name of the Examination :
M. Sc. (Sem. 1)
Name of the Subject:
Chemistry : Paper - 4
-Section No. (1,2......): Nil
Student's Signature
-Subject Code No.
(2) All questions are compulsory.
(3) Figures to right indicate full marks of that question.
(4) Draw neat diagram or figures where necessary.
(a)
18
electronic transitions
n = K
(ii)
(i)
(b)
(ii)
Answer any three :
(i) Explain % and giving examples.
Explain the effect of polar solvents in UV absorption. State and explain Lambert Beers Law. What are its limitations?
Why the increase in polarity of a compound shifts
bands to longer wavelength but n >k and
n>G* bands to shorter wavelength?
Explain the factors which influence the vibrational frequency of a particular functional group. Give examples.
(c) (i)
(ii)
Why does chloroform give prominent bands in the main region of IR spectrum while carbon tetrachloride does not?
How can you distinguish the type of H-bonding involved in a compound by IR spectrum? Describe sampling methods in IR spectroscopy.
(d)
(i)
(e) (i) Which of the following two compounds will have higher A]nax and why?
C6H5-CH=CH-C6H5 and C6H5-CH2-CH=CH-CH2-C6H5 I II
(ii) Explain the terms : Inductive, mesomeric and conjugation effects in IR spectroscopy.
2 (a) Answer any three : 18
(i) Explain the basic principle of separation in TLC.
(ii) In what way TLC is superior to other chromatographic techniques?
(b) (i) Describe three methods of preparation of TLC
plate.
(ii) Explain five applications of TLC.
(c) (i) Mention five widely used stationary phase with its
common trade name and applications in GC.
(ii) Explain the working principle of FID detector used in GC.
(d) (i) Describe direct method of spot detection in a thin
layer chromatography.
(ii) Describe SCOT and WCOT columns used in gas chromatography. Which is better? Why?
(e) (i) Describe the method for injection of samples in GC.
(ii) Write notes on coating materials in TLC and
mobile phase in GC.
3 Answer any three : 18
(a) (i) Distinguish between accuracy and precision with
suitable data of an example.
(ii) Explain how do you perform Q test?
(b) (i) What are systematic and random errors? Explain
with examples.
(ii) Explain significance of confidence limit with illustration.
(c) (i) When do you perform t-test? What information is
obtained?
(ii) State difference between mean and median with data of analysis.
(d) (i) What is the use of least square method? How do
you perform it to obtain data points?
(ii) Explain factors that can minimize errors.
(e) (i) Describe the properties of normal distribution curve.
(ii) Explain the terms : methodical error and
propagation error.
for the given structures :
(a) Calculate \iax
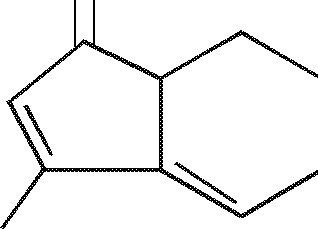 |
|
(i) |
|
and | 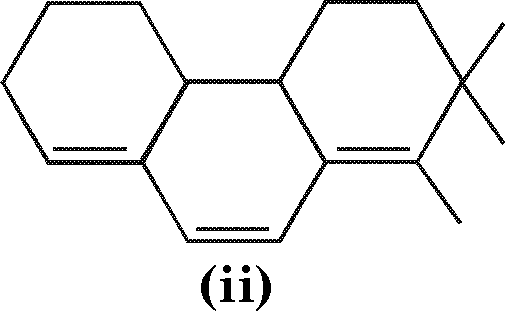 |
Distinguish between each pair in IR spectra :
(b)
(c)
(d) (e)
(i) Cis and trans butene
(ii) Alkyl nitrile and benzonitrile
(i) Discuss the nature of interaction forces on stationary phase for solute.
(ii) Explain elutropic series.
(i) Draw a neat diagram of instrumentation of GC. Mention function of each components.
(ii) Write note on temperature programming.
In an experiment, the percentage of copper in a sample
had the following results : x = 15.30
S = 0.10 n = 4
(i) Calculate the 90% confidence interval of the mean if t = 2.353.
(ii) Calculate 99% confidence interval of the mean if t = 5.841.
S-2604] 3 [ 500 ]
|
Attachment: |
| Earning: Approval pending. |
