Indian Institute of Technology New Delhi - (IIT) 2011 B.Tech with solutions IIT - Question Paper
IIT-JEE 2011 ques. paper with solutions
This is the complete IIT-JEE 2011 ques. paper along with detailed answers, so that it helps a learner to get the answers along with the ques. and would not have to refer any other books or solutions.
The Indian Institutes of Technology (popularly known as IITs) are institutions of national importance established through an Act of Parliament for fostering excellence in education. There are fifteen IITs at present, located in Bhubaneswar, Chennai, Delhi, Gandhinagar, Guwahati, Hyderabad, Indore, Jodhpur, Kanpur, Kharagpur, Mandi, Mumbai, Patna, Ropar and Roorkee. Over the years IITs have created world class educational platforms dynamically sustained through internationally recognized research based on excellent infrastructural facilities. The faculty and alumni of IITs continue on making huge impact in all sectors of society, both in India and abroad. Institute of Technology, Banaras Hindu University (IT-BHU), Varanasi and Indian School of Mines (ISM), Dhanbad, are amongst the oldest institutions in India and are known for their immense contributions towards society at large and for science and technology in particular.
Sri Chaitanya IIT Academy.A.P. >1< IIT JEE 2011 Paper - I
Time: 3 Hours Maximum Marks: 240
Please read the instructions carefully. You are allotted 5 minutes specifically for this purpose
A. General :
1. The question paper CODE is printed on the right hand top corner of this sheet and also on the back page (page no 36 of this booklet)
2. No additional sheets will be provided for rough work
3. Blank papers, clipboards, log tables, slide rules, calculators, cellular phones, pagers and electronic gadgets in any form are not allowed
4. Write your name and registration number in the space provided on the back page of this booklet.
5. The answer sheet, a machine - gradable Objective Response Sheet (ORS), is provided separately.
6. DO NOT TAMPER WITH/MULTILATE THE ORS OR THE BOOKLET.
7. Do not break the seals of the question qpeffeoa'klet before instrucuted to do so by the invigilators.
8. This question paper contains 36 pages haling 69 questions.
9. On breaking the seat, please check that all the questions are legible.
B. Filling the bootom half of the ORS :
10. The ORS has CODE printed on its lower and upper Parts.
11. Make sure the CODE on the ORS is the same as that on this booklet. If the Codes do not match, ask for a change of the Booklet.
12. Write your Registration No., Name and Name of centre and sign with pen in appropriate boxes. Do not write these anywhere else. Darken the appropriate bubbles below your registration number with HB pencil.
C. Question paper format and marking scheme :
13. The question paper consists of 3 Parts (Chemistry, Physics and Mathematics). Each part consists of four sections
14. In Section I (Total Marks : 21), for each question you will be awarded 3 marks if you darken ONLY the bubble corresponding to the correct answer and zero marks if no bubble is darkened. In all other cases, minus one (-1) mark will be awarded.
15. In Section II (Total Marks : 1 6), for each question you will be awarded 4 marks if you darken ALL the bubble(s) corresponding to the correct answer(s) ONLY and zero marks otherwise. There are no negative marks in this section.
16. In Section III (Total marks : 15), for each question you will be awarded 3 marks if you darken ONLY the bubble corresponding to the correct answer and zero marks if no bubble is darkened. In all other cases, minus one (-1) mark will be awarded.
1 7. In Section IV(Total Marks : 28), for each question you will be awarded 4 marks if you darken ONLY the bubble corresponding to the correct answer and zero marks otherwise. There are no negative marks in this section
IIT JEE 2011 (Paper - I) Part - I , PART I : CHEMISTRY
SECTION - I ( Total Marks : 21)
( SINGLE CORRECT CHOICE TYPE )
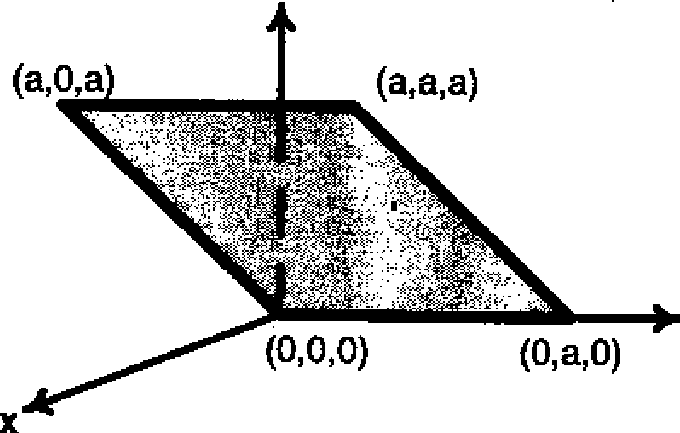
This section contains 7 multiple choice questions. Each question has four choices A,B,C and D out of which ONLY ONE is correct.
m =
 |
|
(S) |
 |
|
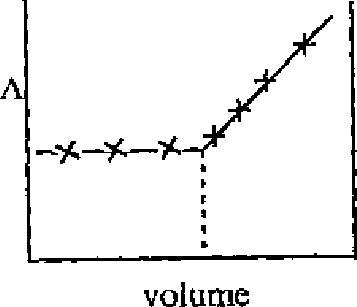
(Q) |
 |
|
(P) |
volume
(R)
c) (R)
a) (P)
Ans : D
b) (Q)
d) (S
Sol : Equation : K+ + Cl + Ag+NO3 K+ + NO3 + AgCl
In the early stages of the titration, addition of silver nitrate, the conductance does not
change very much because the Cl ions are replaced by NO3- ions; both has almost same
ionic conductance. After the end point is passed, the excess of the added salt causes a sharp increase in conductance.
Acr = 76 34 S cm2 mole
AAg+ = 6192 s cm2 mole
ANO- = 7144 S cm2 mole
3. Among the following compounds, the most acidic is
a) p-nitrophenol b) p-hydroxybenzoic acid
c) o-hydroxybenzoic acid d) p-toulic acid
Ans : C
Sol : Ortho effect present in ortho-hydroxy benzoic acid
4. The major product of the following reaction is
(i) KOH
NH
CHC1
a)
Ans : A
|
d) |  |
I > |
O
N K+
O
(ii) Br
 |
b) |
/) CHC1
CHC1
Br-
CH2Cl
Sol :
O
N-CH2_
-Br
O
5. Extra pure N2 can be obtained by heating
c) (NH 4 )2 CrA d) Ba (N3 )2
a) NH3 with CuO b) NH4NO3 Ans : D
Sol : Pure N2 is prepared by heating Ba(N3)2 Ba (N3 )2 3N21+ Ba
6. Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl , CN and H2O, respectively, are
a) octahedral, tetrahedral and square planar
b) tetrahedral, square planar and octahedral
c) square planar, tetrahedral and octahedral
d) octahedral, square planr and octahedral Ans : B
Sol : [NiCl4] 2 = d8 = Sp3Tetrahedral
[NiCN4] 2 = d8 = dsp2 Square planar
[Ni (H2O)6]+2 = d8 = sp3d2 Octahedral Shape
Bombardement of aluminum by a-particle leads to its artifical disintegration in two ways,
7.
en)
(i) and (II) as shown. Products X, Y and Z respectively are,
0 P + Y
(i)
34 Si+x
a) proton, neutron, positron
b) neutron, positron, proton
c) proton, positron, neutron
d) positron, proton, neutro
Ans : A
0
+1K
Sol : X= !h , Y=0n, Z=
SECTION - II ( Total Marks : 16)
( MULTIPLE CORRECT ANSWERS TYPE)
This section contains 4 multiple choice questions. Each question has four choices A,B,C and D out of which ONLY or More may be correct.
8. Amongst the given options, the compound(s) in which all the atoms are in one plane in all the possible conformations (if any), is (are)
H H
\ / /H c _ c _ /
a) /s b) H2C = C = O c) H - C=C - C d) H2C = C = CH2
h2c ch2 CH2
Ans : ABC
Sol : CH2 = C = CH2 allene system the compound is non planar
9. According to kinetic theory of gases
a) collisions are always elastic
b) heavier molecule s.trafmf e momentum to the wall of the container.
ecules transfer more momen
c) only a molecules have very high velocity
d) between collisions, the molecules move in straight line with constant velocities.
Ans : A,B,C,D Sol :Conceptual
10. The correct statement(s) pertaining to the adsorption of a gas on a solid surface is (are)
a) Adsorption is always exothermic
b) Physisorption may transform into chemisorption at high temperature
c) Physisorption increases with increasing temperature but chemisorption decreases with increasing temperature
d) Chemisorption is more exothermic than physisorption, however it is very slow due to higher energy of activation.
Ans : ABD
Sol: Physical adsorption decreases with increasing of temperature according Leachtlier s Principle
11. Extraction of metal from the ore cassiterite involves
a) carbon reduction of an oxide re b) self-reduction of a sulphide ore
c) removal of copper impurity d) removal iron impurity
Ans : ACD
Sol : Sn is extracted by the reduction of SnO2 with carbon
SnO2 + C Sn + 2CO
Sn mainly contains Iron impurities which are removed by blowing air to convert Fe into FeO. Sn also contains tracer of Cu
SECTION - III ( Total Marks : 15)
( PARAGRAPH TYPE)
This section contains 2 paragraphs. Based upon one of the paragraphs 2 multiple choice questions and based on the other paragraph 3 multiple choice questions have to be answered. Each of these questions has four choices A,B,C and D out of which ONLY ONE is cect.
Passage-I:
|
(i) dil. HgSCyHgSCU P (CgHio) (ii) NaBH4/ethanol |
(iii) dil. acid <i) conc. H2S04 (catalytic amount) (-H20) |
O II 2 /Cs H3C ch3 |
An acylic hydrocarbon P, having molecular formula C6H10, gave acetone as the only organic product through the following sequence of reactions, in which Q is an intermediate organic compound.
12. The structure of compound P is

a) CH3CH2CH2CH2 - c=c - h
|
b) h3ch2c - c=c - ch2ch3 | |
|
d) |  |
Ans : D
13. The structure of the compound Q is


b)

OH
d) ch3ch2ch2chch2ch3
Ans : B
Sol for Q no 12 to 13 :
fa*
-C-CH, WN
CH,
CH,
CH, OH
, _(1)NaB4/e,ha"' > CH, - C-CH - CH,
(2)dil .acid I
CH3 - C - CC - H 24/ 4 > CH, - C-
CH,
(P)
CH,
CH,
(Q)
con. H2 SO4 (catalytic amount)
t
CH,
CH,
CH,
CH, - C-CH - CH
CH, - C=C - CH, - CH, - C-CH - CH,
,
CH,
(1) O3
CH,
CH,
( 2 ) Zn / H 2O
<
CH,
2 CH, - C=O
Passage- II :
When a metal rod M is dipped into an aqueous colourless concentrated solution of compound N, the solution turns light blue. Addition of aqueous NaCl to the blue solution gives a white precipitate O. Addition of aqueous NH3 dissolves O and given an intense blue solution
c) Ni
d) Co
c) Al(NO3)
)3
d) Pb(NO3)
3'2
32
&
14. The metal rod M is
a) Fe b) Cu
Ans : B
15. The compound N is
a) AgNO3 b) Zn(NO3)
Ans : A
16. The final solution contains
b) [Al(NH3)4]3+ and [Cu(NH3)4]
a) [Pb(NH3)4]2+ and [CoCl3)4]
c) [Ag(NH3)2]+ and [Cu(NH3)4]2+
d) [Ag(NH3)2]+ and [NiVp
Ans : C
Sol : for Q. no 14 to 16 :
Cu +2Ag NO3 Cu (NO3 )2 + 2Ag
(M)
(N)
NaCl Na+ ( aq) + Cl (aq)
Ag ++ ClAgCl I
White(O)
Cu+2 + 4 (NH3) [Cu (NH3 )4
Deep blue colour
SECTION - IV ( Total Marks : 28)
( INTEGER ANSWER TYPE)
This section contains 7 questions. The answer to each of the questions is a single-digit integer, ranging from 0 to 9. The bubble corresponding to the correct answer is to be darkened in the ORS.
17. The work function (0) of some metals is listed below. The number of metals which will show photoelectric effect when light of 300 nm wavelength falls on the metal is
|
Metal |
Li |
Na |
K |
Mg |
Cu |
Ag |
Fe |
Pt |
W |
|
0( eV ) |
2.4 |
2.3 |
2.2 |
3.7 |
4.8 |
4.3 |
4.7 |
6.3 |
4.75 |
Ans : 4
Sol : E=0+KE
For photo electric effect, condition is E > 0 Metals exhibits : Li, Na, K, Mg
18. To an evacuated vessel waithovable piston under external pressure of 1 atm., 0.1 mol of He and 1.0 mol of an unknown compound (vapour pressure 0.68 atm. at 0 0C) are introduced. Considering the ideal gas behaviour, the total volume (in litre) of the gases at 0 0C is close to
Ans : 7
Sol : Unknon Compound may be Soid (or) liquid Let given volume of vessel : V for unknown compound PV=nRT
0.68 X V = ng (0.0821 X 273) 1
o
For total gaseous mixture pressure = external. pressure PV=nRT
1 X V =(ng + nHe)(0.0821 X 273)_2
from 1 and 2
ng = 0.2125, ntotal = 0.3125
V = 7 lit
Sri Chaitanya IIT Academy.A.P. >10< :: IIT JEE 2011 Paper - I ::
19. Reaction of B with N2CO3 in aqueous solution gives sodium bromide and sodium bromate with evolution of CO2 gas. The number of sodium bromide molecules involved in the balanced chemical equation is
Ans : 5
Sol : 3Br2 + 3Na2CO3 5NaBr + NaBrO3 + 3CO2
20. The difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is Ans : 5
O
O
(0) (0) II
. NaO - S - S - S - S - ONa
S01 : (+5)11 II (+5) **4
O O A0*
The difference between the O.S of two different types qfiuiphur atoms = +5
21. A decapeptide (Mol. Wt. 796) on complete hydrolysis gives glycine (Mol. Wt. 75), alanine and phenylalanine. GlycinecontribuSesft/- 0% to the total weight of the hydrolysed products. The number of glyGinlwiiispresent in the decapeptide is
Ans : 6
Sol : 100_958
47 ? x 958 = 450.12 - ' 100
The weight of glycine present in 958 decapeptide is 450.12.
the molecular weight of one glycine is 75
450.12
no. of glycines = = 6 00 = 6
22. The total number of alkenes possible by dehydrobromination of 3-bromo 3-cyclopentylhexane using alcoholic KOH is
Ans : 5
Sri Chaitanya IIT Academy.A.P. Sol :
|
Br |
 |
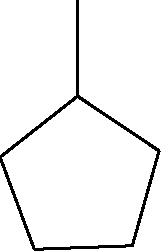 |
|
C - C - C - C = C - C | |
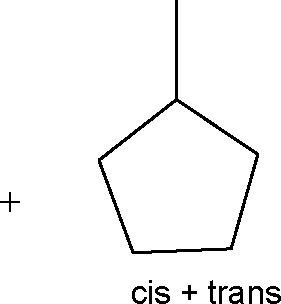 |
+ |
|
C - C - C = C - C - C | |
C - C - C - C - C - C C - C - C - C - C - C c - C - C - C - C - C
cis + trans
%
23. The maximum number of electrons that can have principal quantum number, n=3, and spin quantum number, ms=-1/2, is
Ans : 9
Sol : In third orbit 3s, 3p, 3d sub levels are present and a total of nine orbitals are present. Each orbital has one electron with ms = -1/2
SECTION - I (Total Marks : 21)
( SINGLE CORRECT CHOICE TYPE )
This section contains 7 multiple choice questions. Each question has four choices (A), (B), (C) and (D) for its answer, out of which ONLY ONE is correct
24. The wavelength of the first spectral line in the Balmer series of hydrogen atom is 6561 A,
The wavelength of the second spectral line in the Balmer series of singly-ionized helium atom is
2430 A
a)1215A
c)
d) 4687 A
b) 1640 A
Ans : A
Sol
5 4 x16
x-
12
A
9 x 3
 |
|
c) 27 |
a) 9 Ans : D
b) 18
d) 36
_ 1 1 2
w= 36
w _ 2 x18
22
26. A meter bridge is set-up as shown, to determine an unknown resistance X using a standard 10 ohm resistor. The galvanometer shows null point when tapping - key is at 52 cm mark. The end-corrections are 1 cm and 2cm respectively for the ends A and B. The determined value of X is
 |
|
b) 10.6 ohm c) 10.8 ohm |
a) 10.2 ohm Ans :B
d) 11.1 ohm
10
a
Sol :
(J
2
Y 10
52 +1 48 + 2
A' = 10'v3iJ2
X = 10.6Q
a) 0% Ans : D
|
27. A 2F capacitor is charged as shown in figure. The percentage of its stored energy dissipated after the switch S is turned to position 2 is | |
|
b) | 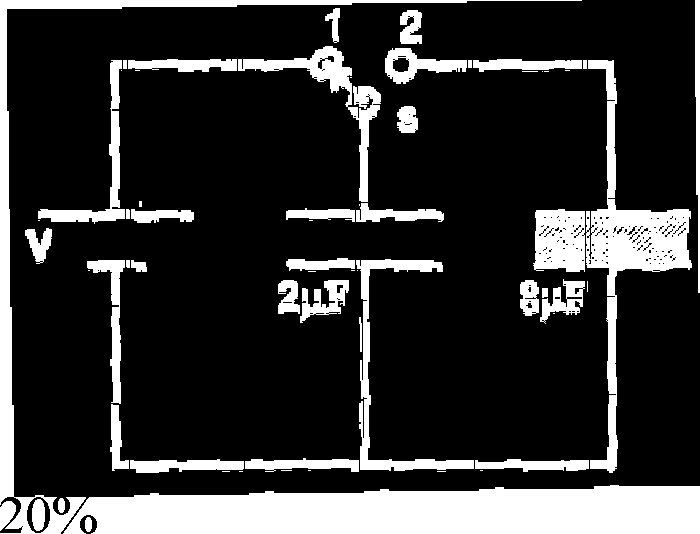 |
|
c) 75% | |
d) 80%
Sol : q0 = 2V
qL= q2 2 8
4q1 = q2 q + q2 = 2V
5q _ 2V q,_( f v ) q =( 5 V)
4
1 4V2 _ 1 25
2 f 2 ;
64 1 25
V2
V2
- +
4V2 = V2 +V2
25
25

4V2 _ 1 -1 -
2 25
4V2 _ 25-1 -4 _ 20x100 = 80 25 25
28. A police car with a siren of freqjftenckHz is moving with uniform velocity 36km/hr towards a tall building which reflects the sound waves. The speed of sound in air is 320 m/s. The frequency of the siren heard by the car driver is
a) 8.50 kHz b) 8.25 kHz c) 7.75 kHz d) 7.50 kHz
Ans : A
Aol : frequency reflucted by wall f
f' _ 8 x103
320
320 -10
frequency heared by car driver f" _
320 +10 320
f'
3 32 33
f" _ 8 x103 x 31 32
33
_ 8 x103 x
31
8.50 kHz
29. 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 liter. Taking the initial temperature to be T, the work done in the process is
9
a) 8 RT
c) 15 RT
9
d) 2 RT
b) 2 RT
Ans: A
R
Sol: W = -(T - 72)
TlVlr = k
\7
t vr=t2 i 8
T2 = 471
9 RT
W=
8
30. Consider an electric field E = E0x, where kP*a constant. The flux through the shaded area (as shown in the figuieiSuPfiothis field is
: Epia const
 |
y |
|
a | |
E0a
7T
b) V2ec
a) 2E0 a2 Ans : C
c) E0 a2
d)
z

area = 42a2 EA = E2a2 cos 450 = Ea2
SECTION - II (Total Marks : 16)
( MULTIPLE CORRECT CHOICE TYPE )
This section contains 4 multiple choice questions. Each question has four choices (A), (B), (C) and (D) for its answer, out of which ONE or MORE may be correct
31. A metal rod of length L and mass m is pivoted at one end. A thin disk of mass M and radius R(<L) is attached at its center to the free end of the rod. Consider two ways the disc is attached; (case A) the disc is not free to rotate about its center and (case B) the disc is free to rotate about its center. The rod - disc system performs SHM in vertical plane after being released from the same displaced position. Which of the following statement(s) is (are) true ?

a) Restoring torque in case A = Restoring torque in case B
b) Restoring torque in case A < Restoring torque in case B
c) Angular frequency for case A > Angular frequency for case B
d) Angular frequency for case A < Angular frequency forcase B
ncy for cas
Ans : AD
|
Sol : Torque about point of suspenstion is same in both case | |
|
case A : T = |  |
|
a = kd | |
case B ; In case B ; Disc is not going to get any torque about its centre.
all of its point have acceleration that is equal to acceleration of end point of rod.
a = k6
T =
|
///// |
 |
3
k
k
W =
MR2 , ml2
-+ ML +-
2
3
w2 > w
32. A composite block is made of slabs A, B, C, D and E of different thermal conductivities (given in terms of a constant K) and sizes (given in terms of length, L) as shown in the figure. All slabs are of same width. Heat Q flows only from left to right through the blocks. Then in steady state

a) heat flow through A and E slabs are same
b) heat flow through slab E is maximum
c) temperature difference across slab E is smallest
st
d) heat flow through C = heat flow through B + heat flow through D
Ans :ABCD
4/3
1/6 /WVw
i, 1/2
Sol : ,= | = 0.57
1/6
JWVu
h = 1.27
1/4
JWW.
1/12 TsAW-
O
T
i3 - 0.77
*2 i1 i3
1/6
-NWU
4/5
1/2
33. An electron and a proton are moving on straight parallel paths with same velocity. They enter a semi-infinite region of uniform magnetic field perpendicular to the velocity. Which of the following statement(s) is/are true ?
a) They will never come out of the magnetic field region
b) They will come out travelling along parallel paths
c) They will come out at the same time
d) They will come out a different times
Ans : BD
mv
So' :r _ qB
They go in different direction but come out along parallel paths.
T _ 2nm
qB
as m is different T also different
34. A spherical metal shell A of radius RA and a solid metal sphere B of radius RB (< RA) are
kept far apart and each is given charge +Q. Now they are connected by a thin metal wire. Then
a) Eide _ 0 b) Qa > Qb c) g_ d) EAnsurface < E0Bnsurface
Ans :ABCD
Sol : a) Ea = 0
,ft _ Qbl
b) Ra Rb
RA > RB
Qa > Qb
c) R _ V
d) E _
2s0
so Ea
SECTION - III (Total Marks : 15)
(COMPREHENSION TYPE)
This section contains 2 paragraphs. Based upon one of the paragraphs 2 multiple choice questions and based on the paragraph 3 multiple choice questions have to be answered. Each of these questions has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct.
Paragraph for Question Nos. 35 to 36
A dense collection of equal number of electrons and positive ions is called neutral plasma. Certain solids containing fixed positive ions surrounded by free electrons can be treated as neutral plasma. Let N be the number density of free electrons, each of mass m. When the electrons are subjected to an to electric field, they are displaced relatively away from the heavy positive ions. If the electric field becomes zero, the electrons begin to oscillate about the positive ions with a natural angular frequency 'op' which is called the palsma frequency. To sustain the oscillations, a time varying electric field needs to be applied that has an angular frequency o, where a part of the energy is absorbed and a part of it is reflected. As o approaches op, all the free electrons a set to resonance together and all the energy is reflected. This is the explanation of hjgh reflectivity metals.
35. Taking the electric charge as 'e' and the permittivity as 's0', use dimesional analysis to determine the correct expression for op.
p
Ne2
Ne
ms
ms0
'Ne2
a)
c)
d)
ms,
Ans : C
n
Sol : o _ t = T-
1
Ne2
D.f. of
x IT2 x M lL3T -2
msn
D.f. = T-i
36. Estimate the wavelength at which plasma reflection will occur for a metal having the density of electrons N - 4 x 1027 m 3. Take s0 -10-11 and m -10-30, where these quantities are in proper SI units.
a) 800 nm b) 600nm c) 300nm d) 200nm
Ans : B
o
Sol : o _ 2nn n _ 2n
, , c c x 2 , c x 2n
c = nA A = =- A = -
3x10 x2n
Ne2
n
a
1
msn
4x1027 x(1.6x10-19)2
10-30 x10-11
Paragraph for Question Nos. 37 to 39
Phase space diagrams are useful tools in analyzing all kinds of dynamical problems. They are especially useful in studying the changes in motion as initial position and momentum are changed. Here we consider some simple dynamical systems in one-dimension. For such systems, phase space is a plane in which position is plotted along horizontal axis and momentum is plotted along vertical axis. The phase space diagram is x(t) vs. p(t) curve in this plane. The arrow on the curve indicates the time flow. For example, the phase space diagram for a particle moving with constant velocity is a straight line as shown in the figure. We use the sign convention in which position or momentum upwards (or to right) is positive and downwards (or to left) is negative
e
o
S
o
S
Position
37. The phase spacem diagram for a ball thrown vertically up from ground is
|
Momentum |
 |

|
b) |  |
|
d) | 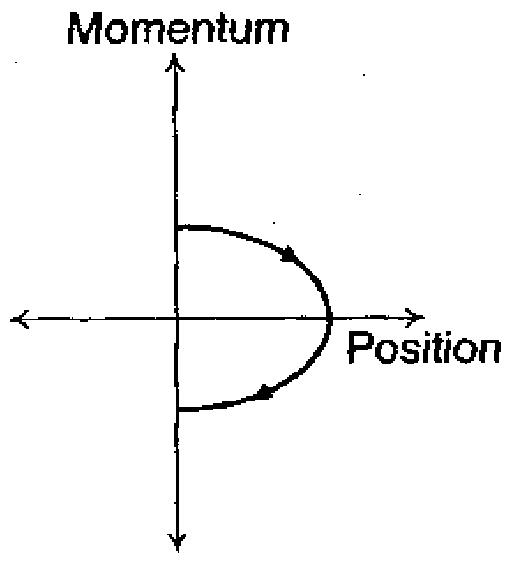 |
Ans : D
Sol : Vertically thrown particle comes back to original position till the particle reaches the highest position of its path, its momentum is +ve. After that, it terns to zero and them negative
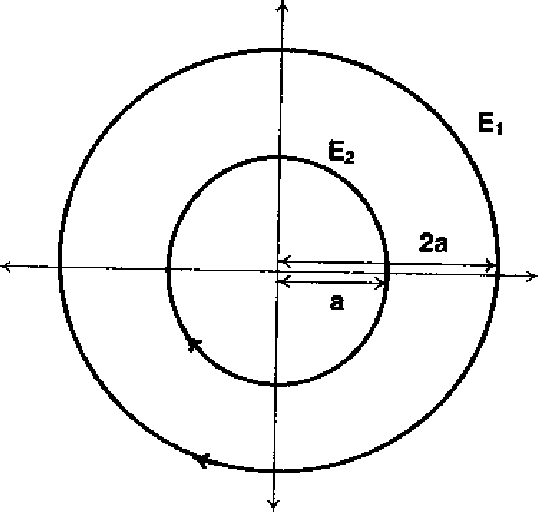
38. The phase space diagram fro simple harmonic motion is a circle centrred at the origin. In the figure, the two circles represent the same oscillator but for different initial conditions, and E1 and E2 are the total mechanical energies respectively. Then
Momentum
 |
Position |
|
c)Ej =4 E2 | |
b) Ej =2 E2
d) Ej =16 E2
a) Ej = V2 e2 Ans : C
Sol : Energy = 1 ka2 a a2
El = 0l = 4 E2 a2
E1 = 4E2
4
thespring-mass system, with the mass submerged in water,
39. Consider lrra'gnriig-uiaaa aaicui, wiui uic uiaaa auuuicigcu m vvaiLi, _awss
as shown iiUne figure. The phase space diagram for one cycle of this system is
|
b) |  |


|
d) |  |
Ans : A
Sri Chaitanya IIT Academy.A.P. >22< :: IIT JEE 2011 Paper - I ::
Sol : As the block oscillates, due to viscous effects, its total energy decreases continuously and so its amplitude decreases continuously. Assuming that the block is initially pulled down and released, its momentum will increase upwards till it reaches the mean position
SECTION -IV
( INTEGER ANSWER TYPE)
This section contains 7 questions . The answer to each of the questions is a single -digit integer, ranging
from 0 to 9. The bubble corresponding to the correct answer is to be darkened in the ORS.
40. Steel wire of length L at 40C is suspended from the ceilling and then a mass m is hung from its free end. The wire is cooled down from 40C to 30C to regain its original length L. The coefficient of liner thermal expansion of the steel is 10-5/C, Youngs modulus of steell is 1011 N/m2 and radius of the wire is 1mm. Assume that L>> diameter of the wire. Then the value of m in kg is nearly
Ans : 3
mgL
Sol : Y= , e=aL At Ae
Y = . mSL
AaLAL
M x10
1011 =
x1x10 x10" m = 3.14 * 3
41. The activity of a freshly prepared radioactive sample is 1010 disintegrations per second, whose mean life is 109s. The mass of an atom of this radioisotope is 10-23 kg. The mass (in mg) of the radioactive sample is
Ans : 1
Sol : 1010 = x N
:.N = 1019 total mass
= 1025 x1019 kg = 10 6 kg = 1 mg
42. A block moving on an inclined plane making an angle 45 with the horizontal and the coefficient of friction is . The force required to just push it up the inclined plane is 3 times the force required to just prevent from sliging down. If we define N = 10, then N is
Ans : 5
mg mg
Sol :

3 3u = 1 + u 4/u = 2
U = 1/2 10u = 5
A boy is pushing a ring of mass 2 kg and radius 0.5 m with a stick a shown in the figure. The stick applies a force of 2 N on the ring and rolls it without slipping with an acceleration of 0.3m/s2. The coefficient of friction between the ground and the ring is large enough that olling always occurs and the coefficient of friction between the Jjifk and the ring is (P/ 10). The value of P is
43.
|
Stick |
 |
4
Ans Sol :
F = N2+ 2N2 (i)

rp = NR /uNR = 2mR2 a j (ii)
solving (i) and (ii),
= 2.6 and 0.36
but = 2.6 is not a possible solution = 0.36
p = 3.6 * 4
N = 9
A long circular tube of length 10 m and radius 0.3 m carries a current I along its curved surface as shown. A wire-loop of resistance 0.005 ohm and of radjus0.p is placed inside the tube with its axis coinciding with the axis of theJy|!jiTne current varies as
I = I0cos(300 t) where I0 is constant. If th e magnet ic moment of the loop is N010 sin (300 t), then N is
Four solid spheres each of diameter -J5 cm and mass 0.5 kg are placed with their centers at the corners of a square of side 4cm. The moment of inertia of the system about the diagonal of the square is N x 10-4 kg - m2, then N is
: 9
2 2
4 x MR + 2 x M 5
x10-2 V 2 J
N11 x10-4
A

44.
Ans Sol :
45.
Y ,V2 J
= I
2
2
f 4 x10-21
~w
4 x x-5 x 5
+ 2 x-5 x

Ans Sol :
6
. I
m = l
B = 010 cos
300t
l
M010 (300)
dt
sin300t nR2
V =
l
iu010nR2 (300) .
V = -V-Zsin300t
l
. = V
1 = r m = inR2
46. Four point chargs, each of +q, are rigidly fixed at the four corners of a square planar soap film of side a . The surface tension of the soap film is y. The system of charges and
1/N
planar film are in equilibrium, the a
q_
Y
where k is a constant. Then N is
Ans : 3
\|
Sol
a
q
q
2 Aq2 2y[2q2
( aV2 )
- +
_ ya
a
&
N _ 3
SECTION - I (Total Marks : 21)
(Single Correct Answer Type)
This section contains 7 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct
47. Let P = {# : sin#-cos# = V2cos#j and Q = {#: sin# + cos# = V2sin#| be two sets. Then
b) Q P
d) P = Q
c) P Q Ans: D
Sol: P = sin#- cos# =V2cos# sin# = V2cos# + cos# sin # = (V2 +1) cos#
1) cos#
.
V2sin# = cos# + sin#
P = Q
48. Let the straight line * = b divide the area enclosed by y = (1 - x2), y = 0, and * = 0 into two parts R1 (0 < x < b) and R2 (b < x < 1) such that R1 - R2 = 4. Then b equals
3
1
1
a)
c)
d)
4
3
4
b)
2
1
Ans : B
b 1 1 Sol : j(1 -x)2dx-j(1 -x)2dx = 4
Sri Chaitanya IIT Academy.A.P. >27< :: IIT JEE 2011 Paper - I ::
49. Let a and ( be the roots of x2 -6x-2 _ 0, with a > ( . If an _an -(n for n > 1, then the
aw - 2as value of 2a is
a) 1 b) 2 c) 3 d) 4
Ans : C
Sol : a10 _a10 -( _ (a + ()(a9 -(9)-a((a8 -(8)
a10 _ 6a9 + 2a8
50. A straight line L through the point (3,-2) is inclined at an angle 600 to the line + y _ 1. also intersects the x-axis, then the equation of L is
a) y + V3x + 2 - 343 _ 0 b) y-V3x + 2 + 33 _ 0_
c) y - x + 3 + 2>/3 _ 0 d) 43y + x - 3 j Ans : B
Sol : Let m be the slope of the required lin
ne
iHT
m _ 0 or m_
51. Let (x0, y0) be the solution of the following equations
(2x)ln2 _(3y)h3
3lnx _ 2y
Then x0 is
a) 6 b) 3 c) 1 d) 6
Ans : C
Sol : log2 (log2 + log x)_ log3 (log3 + log y) --------1
log 3 (log x) _ log 2 (log y).............2
From 1 & 2
1
log x _- log 2 x _ 2
\/ln3 2
x sin x
C x sin x
52. The value of J /, . dx is ''-sinx + sin(ln6 x )
ln2
13 13 3 13
a) -ln- b) -ln- c) ln- d) -ln-
; 4 2 ; 2 2 ; 2 ; 6 2
Ans : A
Sol : Put x2 = t
i log3 = if
sin t
dt
2. sin t
2 iog2 sin t + sin (log b t)
53. Let a = i + j + k, b = i j k and c = i j k be three vectors. A vector V in the plane of a
and b, whose project, is given by
a) i 3j + 3k b) 3i 3 j k c) 3i j + 3k d) i + 3 j 3k
Ans : C
So1 : Let v = a + Ab Projection V on C
A = 2
Sri Chaitanya IIT Academy.A.P. >29< :: IIT JEE 2011 Paper - I ::
SECTION - II (Total Marks : 16)
(Multiple Correct Answer Type)
This Section contains 4 multiple choice questions. Each question has four choices (A), (B), (C) and (D) out of which ONE or MORE may be correct.
54. L et f : f: K K be a function such that f(x + y) = f(x) + f(y), Vx, y e K .
If f(x) is differentiable at x = 0, then
a) f(x) is differentiable only in a finite interval containing zero
b) f(x) is continuous Vx e K
c) f' (x) is constant Vx e K
d) f(x) is differentiable except at finitely many points B C
Sol : From the given data f (x) be the linear functi|n.|
55. Let the eccentiricty of the hypgbQalix = 1 be reciprocal to that of the ellipse x2 + 4y2 = 4. If the hyperbola passes thorugh a focus of the ellipse, then
i 2 2
a) the equation of the hyperbola is --- = 1
b) a focus of the hyperbola is (2, 0)
c) the eccentricity of the hyperbola is J3
d) the equation of the hyperbola is x2 - 3y2 = 3 Ans : B,D
Sol : Ecentricity of ellipse
Ecentricity of Hyper bola = 3
Focus of ellipse = (V3,0), (V3, 0)
Focus lies on the hyper bola then a = J3, b = 1
56. Let M and N be two 3 x 3 non - singular skew - symmetric matrices such that MN = NM. If PT denotes the transpose of P, then M2N2(MTN)-1(MN-1)T is equal to
a) M2 Ans : wrong
Reason : 3x 3 non singular skew -symmetrices doest exist.
Sol : Among the given options option C may be correct.
57. The vector(s) which is/are coplanar with vectors i + j - 2k and i + 2 j + k, and perpendicular to the vector i + j + k is /are
c) -M2
b) -N2
d) MN
a) j - k b) -i + j
d) - j + k
c) i - j
Ans : A,D
Sol: Required vector = |( a x 6) x c j
(Paragraph Type)
This section contains 2 paragraphs. Based upon one of the paragraphs 2 multiple choice questions and based on the other paragraph 3 multiple choice questions have to be answered. Each of these questions has four choices (A), (B), (C) and (D) out of with ONLY ONE is correct.
Paragraph for Question Nos. 58 and 59
Let U1 and U2 be two urns such that U1 contains 3 white and 2 red balls, and U2 contains only
1 white ball. A fair coin is tossed. If head appears then 1 ball is drawn at random from U1 and put into U2. However, if fail appears then 2 balls are drawn at random from U1 and put into U2. Now 1 ball is drawn at random from U2.
58. The probability of the drawn ball from U2 being white is
13
30
23
30
19
30
11
30
a)
b)
c)
d)
Ans : B
|
Probability = 2 |
|
3C2 3 2C2 1 3C.2C, 2 -x- + 2 x- + 1-Lx 5C2 3 5C2 3 5C |
2
23
30
59. Given that the drawn ball from U2 is white, the probability that head appeared on the coin is
17
11
23
15
23
12
23
a)
b)
c)
d)
23
Ans : D
3 2 2 1 x + x 5 2 5 2
Sol : Required conditional Probability =
23
30
Paragraph for Question Nos. 60 and 62
Let a, b and c be three real numbers satisfying
1 9 7 8 2 7 7 3 71% \
[ a b c ]
[0
(E)
60. If the point P(a, b, c), with reference to (E), lies on the plane 2x + y + z = 1, then the value of 7a + b
a) 0
Ans : D
c) 7
d) 6
61. Let o be a solution of x3 - 1 = 0 with Im( o )>0. If a = 2 with b and c satisfying (E), then
313 the value of + r + is equal to
o o o
a) -2
Ans : A
b) 2
c) 3
d) -3
62. Let b = 6, with a and c satisfying (E). If a and fi are the roots of the quadratic equation
1 1
+ bx + c = 0, then +
n=0 V a
ax
6
a) 6
Ans : B
b) 7
c)
d)
7
TO
Sol : 60, 61, 62.
a + 8b + 7 c = 0 9a + 2b + 3c = 0 a + b + c = 0
a = x, b = 6X, c = -7X
60. Now 2a + b + c = 1 2X + 6X- 7X = 1 X = 1
Hence a = 1, b = 6, c
= -7
.. 7 a + b + c = 6
61. a = 2 X = 2 b = 12, C = -14
3 1 3
--+--b +--3 = 2
Wa Wb W
62. b = 6 X = 1
a = 1, c = 7
ax2 + bx + c = 0 x2 + 6x 7= 0 a = 1, (=-7
y
= 7
SECTION - IV (Total Marks : 28)
(Integer Answer Type)
This section contains 7 questions. The answer to each of the questions is a single - digit integer, ranging from 0 to 9. The bubble corresponding to the correct answer is to be darkened in the ORS.
xf 1 ( x )- f ( x ) = x" f1 ( x )--f ( x ) = x
If =1
x
f ( x). = |1dx f ( x )_
= x + c
n
f ( x ) = x2 + c f (1) = 2
2 = 1 + c
c = 1
f (x) = x2 + x f ( x ) = 4 + 2 =
%
64. If z is any complex numberaying \z - 3 - 2i| < 2, then the minimum value of |2 z - 6 + 5i\
is
Ans : 5
x) x
z - 3 - 2i + 2i +
Sol : 2
= 2
2
> 2 1z - 3 - 2i -
>
> 5
65. Let a1, a2, a3,..........,a100 be an arithmetic progression with a1 = 3 and SP = ai, 1 < P <
100
i=1
S
For any integer n with 1 < n < 20, let m = 5n. If s does not depend on n, then a2 is
Ans : 9
s y[6+(m - 1)d]
m __Z_
So1 : S Hr, ( j|
n 2[6 + (n -1)d]
5 [6 + ( 5n -1) d ]
6 + (n -1) d
Which is independed of n if d _ 6a1 _ 6
a2 _ a1 + d _ 9
66. Consider the parabola y2 = 8x. Let A1 be the area of the triangle formed by the end points of its latus rectum and the point P , 2 j on the parabola, and A2 be the area of the triangle
formed by drawing tangents at P and at the end points of the|latu|jiThen A is Ans : 2
f the lat
.
Sol : Ai _8a!(y'-y2)(y*-y3)(y-
A _ 1
1 16a,1
.A _ 2
A,
-1 sintf U U / s (n\\
67. Let f (n)_sin tan I , where -< n < . Then the value of d(tnn) (f (n)) is
Vcos2n JJ 4 4 d (tann)
V v
Ans : 1
Sol : Put tan
_ a
Vcos20
sinn
tana _ --
Vcos2n
sina _ tann
. f (n)_d(tan)t (n)_1
68. The minimum value of the sum of real numbers a-5, a-4, 3a-3, 1, a8 and a10 with a > 0 is Ans : 8
Sol : AM > GM
1 1 1 1 1 , 8 10
5 + +3 +3 +3 +1 + a + a a a a a a
>881 >8
1 1 1
+ -
2n
3n
n
69. The positive integer value of n > 3 satisfying the equation sin j sin j sin ( I is
Ans : 7
0. 2n 0. 3n 0. n
2n 3n
Sm--+ Sm
So1 : Sm .Sm = Sm
n
n
n
nn
n 5n n 3n 2n 4n
cos--cos = cos--cos + cos--cos
n n n n n n
3n 5n 2n 4n
cos--cos = cos--cos
n n n n
"fllSP
4n n 3n
2sin sin = 2sin si nn
nn
n
sin 4
%
n
2cos I I sin I 1 = 0
I2nJ I 2nJ
7 n
cos = 0 (.-. n>3) 2n
7n n
= ( 2k +1)
2n 2
= odd integer n
Sri Chaitanya IIT Academy, Plot No 512, Ayyappa Society, Madhapur, Hyderabad - 500081
@: 040-23119393, Website : www.srichaitanya.net
Dissolving 120 g of urea (mol. wt. 60) in 1000g of water gave a solution of density 1.15 g/mL. The molarity of the solution is
a) 1.78 M b) 2.00M c) 2.05 M d) 2.22 M
Ans : C
Sol : Molarity = 2m
1000M
ld(g/cc) - MMWSolute
M = 2.05M
9 X 3
A ball ofmass (m) 0.5 kg is attached to the end of a string having length (L) 0.5 m. The ball is rotated on a horizontal circular path about vertical axis. The maximum tension that the string can bear is 324 N. The maximum possible value of angular velocity of ball (in radian/s) is
ml2
mL +
|
Attachment: |
| Earning: Approval pending. |
